Chapter #14 Newest CD
... CnH2n+2 Compounds containing only carbon and hydrogen with only single bonds and no multiple bonds, but a ring structure - Saturated hydrocarbons - Cycloalkanes CnH2n Compounds containing only carbon and hydrogen with double bonds - Unsaturated hydrocarbons - Alkenes CnH2n Compounds containing only ...
... CnH2n+2 Compounds containing only carbon and hydrogen with only single bonds and no multiple bonds, but a ring structure - Saturated hydrocarbons - Cycloalkanes CnH2n Compounds containing only carbon and hydrogen with double bonds - Unsaturated hydrocarbons - Alkenes CnH2n Compounds containing only ...
carboxylic acids and their derivatives
... be extracted from cells and tissues by nonpolar solvents (chloroform, ether, benzene). Compounds that meet this definition are substantially hydrocarbonlike, although they may differ widely in structure. They include not only esters of long-chain fatty acids but steroids (Section 30-4), terpenes (Se ...
... be extracted from cells and tissues by nonpolar solvents (chloroform, ether, benzene). Compounds that meet this definition are substantially hydrocarbonlike, although they may differ widely in structure. They include not only esters of long-chain fatty acids but steroids (Section 30-4), terpenes (Se ...
top organomet chem-2006-19-207 pauson
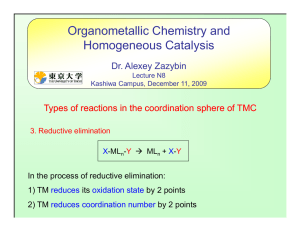
... (CO)2 ]2 [90], Jeong has introduced several species as new catalysts. Some of these rhodium complexes need activation with AgOTf. The reaction works well with non-terminal alkynes (36) and the scope and efficiency is dependent on the catalyst used. In the case of chiral species, a careful choice of c ...
... (CO)2 ]2 [90], Jeong has introduced several species as new catalysts. Some of these rhodium complexes need activation with AgOTf. The reaction works well with non-terminal alkynes (36) and the scope and efficiency is dependent on the catalyst used. In the case of chiral species, a careful choice of c ...
C1 polymerization and related C-C bond forming - UvA-DARE
... transition metal catalyzed ethene polymerization to linear polyethylene (which was discovered more than half a century later).10-12 Therefore, most reported C1 polymerization techniques involve the more practical use of a Lewis acid or transition metal (TM) catalyst to polymerize the diazo compounds ...
... transition metal catalyzed ethene polymerization to linear polyethylene (which was discovered more than half a century later).10-12 Therefore, most reported C1 polymerization techniques involve the more practical use of a Lewis acid or transition metal (TM) catalyst to polymerize the diazo compounds ...
General and Selective Synthesis of (Z)-3
... (Z)- and (E)-isomers was obtained by decreasing the amount of MeOH to 0.2 mL (entry 3).9 Encouraged by the results, we then examined a series of solvents (entries 3-6), and DCE (1,2-dichloroethane) was the most effective (entry 7). In the presence of 5 mol % of PdBr2 and 5 equiv of CuBr2, treatment ...
... (Z)- and (E)-isomers was obtained by decreasing the amount of MeOH to 0.2 mL (entry 3).9 Encouraged by the results, we then examined a series of solvents (entries 3-6), and DCE (1,2-dichloroethane) was the most effective (entry 7). In the presence of 5 mol % of PdBr2 and 5 equiv of CuBr2, treatment ...
Lecture8
... N-oxides (R = Me or Et) is commonly used instead of heat or UV-irradiation to remove CO ligands in order to speed up dissociative substitution reactions. ...
... N-oxides (R = Me or Et) is commonly used instead of heat or UV-irradiation to remove CO ligands in order to speed up dissociative substitution reactions. ...
Handout VI
... amine often leads to polyalkylated amines. In case of methylation, the methylation can be stopped after one methyl group is added to the amine by treating it with formaldehyde to form an enamine followed by reduction (Scheme 5). H3C ...
... amine often leads to polyalkylated amines. In case of methylation, the methylation can be stopped after one methyl group is added to the amine by treating it with formaldehyde to form an enamine followed by reduction (Scheme 5). H3C ...
Full Text - Journal of the Indian Institute of Science
... commonly employed for this transformation, are often inappropriate in the presence of other functional groups and therefore require protection or an entirely alternative synthesis. Burgess reagent can, however, be used efficiently for this transformation (eqn 20). ...
... commonly employed for this transformation, are often inappropriate in the presence of other functional groups and therefore require protection or an entirely alternative synthesis. Burgess reagent can, however, be used efficiently for this transformation (eqn 20). ...
23.2 Alcohols, Ethers, and Amines
... Diethyl ether was the first reliable general anesthetic. • However, because diethyl ether is highly flammable and often causes nausea, it was eventually replaced by other anesthetics. ...
... Diethyl ether was the first reliable general anesthetic. • However, because diethyl ether is highly flammable and often causes nausea, it was eventually replaced by other anesthetics. ...
dr.ebtehal Lec3
... o Types of organic reaction ** Elimination ** Addition ** Rearrangement ** Free radical ...
... o Types of organic reaction ** Elimination ** Addition ** Rearrangement ** Free radical ...
Nomenclature Chapter
... R = any general carbon group (it sometimes includes hydrogen too) Ar = any general aromatic group, (when more specificity than ‘just’ R is desired) The foundation of organic nomenclature requires an ability to name alkanes, alkenes and alkynes. Learning the rules for these groups will be your bigges ...
... R = any general carbon group (it sometimes includes hydrogen too) Ar = any general aromatic group, (when more specificity than ‘just’ R is desired) The foundation of organic nomenclature requires an ability to name alkanes, alkenes and alkynes. Learning the rules for these groups will be your bigges ...
Changing counterion can switch the preference for selective 1,2
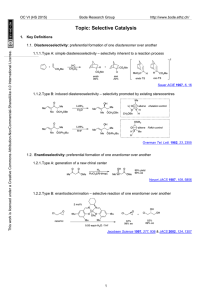
... Another example of a selectivity-controlled reaction of alkenes is hydroformylation via the use of scaffolding ligands. These bind covalently and reversibly to the substrate, leading to a temporarily intramolecular transformation that can lead to dramatically improved and reversed selectivity with s ...
... Another example of a selectivity-controlled reaction of alkenes is hydroformylation via the use of scaffolding ligands. These bind covalently and reversibly to the substrate, leading to a temporarily intramolecular transformation that can lead to dramatically improved and reversed selectivity with s ...
CHM 103 Lecture 23 S07
... Solubility of Alcohols & Ethers in Water Alcohols and ethers • are more soluble in water than alkanes because the oxygen atom can hydrogen bond with water. • with 11-4 C atoms are soluble, but not with 5 or more C atoms. ...
... Solubility of Alcohols & Ethers in Water Alcohols and ethers • are more soluble in water than alkanes because the oxygen atom can hydrogen bond with water. • with 11-4 C atoms are soluble, but not with 5 or more C atoms. ...
organic practice problems
... c. Both diamond and graphite are very hard. d. Both diamond and graphite are very soft. 9. What do all organic compounds contain? a. hydrogen c. oxygen b. water d. carbon 10. Which of the following is an atom or a group of atoms responsible for the specific properties of an organic compound? a. isom ...
... c. Both diamond and graphite are very hard. d. Both diamond and graphite are very soft. 9. What do all organic compounds contain? a. hydrogen c. oxygen b. water d. carbon 10. Which of the following is an atom or a group of atoms responsible for the specific properties of an organic compound? a. isom ...
university of london thesis
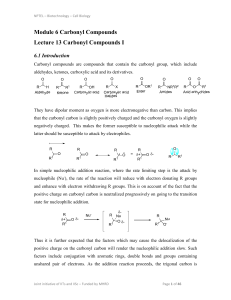
... an oxygen and two carbon atoms, and are among the most intensively studied functional groups. They are commonly used as synthetic intermediates due to their facile preparation from a variety o f starting materials, often with substantial stereochemical control, and because o f their high reactivity ...
... an oxygen and two carbon atoms, and are among the most intensively studied functional groups. They are commonly used as synthetic intermediates due to their facile preparation from a variety o f starting materials, often with substantial stereochemical control, and because o f their high reactivity ...
Project Overview
... Professor William Tam received his B.Sc. at the University of Hong Kong in 1990 and his Ph.D. at the University of Toronto (Canada) in 1995. He was an NSERC postdoctoral fellow at the Imperial College (UK) and at Harvard University (USA). He joined the Department of Chemistry at the University of Gu ...
... Professor William Tam received his B.Sc. at the University of Hong Kong in 1990 and his Ph.D. at the University of Toronto (Canada) in 1995. He was an NSERC postdoctoral fellow at the Imperial College (UK) and at Harvard University (USA). He joined the Department of Chemistry at the University of Gu ...
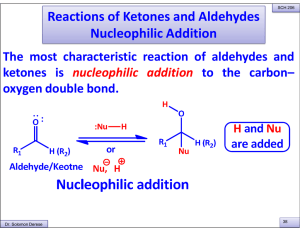
Reactions of Ketones and Aldehydes Nucleophilic Addition
... basic conditions required for anion formation nor survive in a solution containing carbanions. Acetylide ions add to carbonyl groups . The strategy that is routinely followed is to protect the carbonyl group during the reactions with which it is incompatible and then to remove the protecting group i ...
... basic conditions required for anion formation nor survive in a solution containing carbanions. Acetylide ions add to carbonyl groups . The strategy that is routinely followed is to protect the carbonyl group during the reactions with which it is incompatible and then to remove the protecting group i ...
Catalytic, Enantioselective Alkylations of N,O- and
... Conclusion. In summary, we have developed the first practical method for catalytic, enantioselective alkylation of N,O- and N, N-acetals. Stable, readily available acetals la-i can be alkylated with a variety of nucleophiles in up to 96% ee with as little as 1 mol % of catalyst 2. This research has ...
... Conclusion. In summary, we have developed the first practical method for catalytic, enantioselective alkylation of N,O- and N, N-acetals. Stable, readily available acetals la-i can be alkylated with a variety of nucleophiles in up to 96% ee with as little as 1 mol % of catalyst 2. This research has ...
One Step Formation of Propene from Ethene or Ethanol through
... complexes. At the moment, no reports claim nickel ion as a catalytically active species for metathesis. It is noteworthy that the surface density of Ni is approximately 0.5 Ni/nm2 in the case of Ni-M41(20) on the assumption of the even distribution of nickel on the surface. The valence of nickel ion ...
... complexes. At the moment, no reports claim nickel ion as a catalytically active species for metathesis. It is noteworthy that the surface density of Ni is approximately 0.5 Ni/nm2 in the case of Ni-M41(20) on the assumption of the even distribution of nickel on the surface. The valence of nickel ion ...
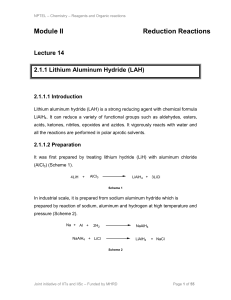
Module II Reduction Reactions
... The reduction of aromatic compounds to 1,4-cyclohexadiene compounds in presence of alkali metal liquid ammonia and an alcohol is called Birch reduction. A variety of aromatic compounds containing electron donating or electron withdrawing groups could be readily converted to the corresponding 1,4cycl ...
... The reduction of aromatic compounds to 1,4-cyclohexadiene compounds in presence of alkali metal liquid ammonia and an alcohol is called Birch reduction. A variety of aromatic compounds containing electron donating or electron withdrawing groups could be readily converted to the corresponding 1,4cycl ...
REDUCTIONS AND REDUCING AGENTS
... ketone with lithium or sodium in liquid ammonia at low temperature. Usually one equivalent of an alcohol or NH4Cl is added to the reaction medium at the end to serve as a proton source / donor to the enolate. ...
... ketone with lithium or sodium in liquid ammonia at low temperature. Usually one equivalent of an alcohol or NH4Cl is added to the reaction medium at the end to serve as a proton source / donor to the enolate. ...
Naming Aldehydes & Ketones
... • Unlike alcohols, aldehydes and ketones cannot hydrogen-bond to themselves, because no hydrogen atom is attached to the oxygen atom of the carbonyl group. • Aldehydes and ketones, therefore, have lower boiling points than alcohols of ...
... • Unlike alcohols, aldehydes and ketones cannot hydrogen-bond to themselves, because no hydrogen atom is attached to the oxygen atom of the carbonyl group. • Aldehydes and ketones, therefore, have lower boiling points than alcohols of ...
HMDS+TMCS+Pyridine - Sigma
... with products containing epoxide rings. Some analysts separate the salt by allowing it to settle, or by centrifuging the material and removing the supernate. Tallent, et al., (3) dissolve the silyl compound in hexane and wash it with water. Formation of ammonium chloride can be avoided by using trif ...
... with products containing epoxide rings. Some analysts separate the salt by allowing it to settle, or by centrifuging the material and removing the supernate. Tallent, et al., (3) dissolve the silyl compound in hexane and wash it with water. Formation of ammonium chloride can be avoided by using trif ...
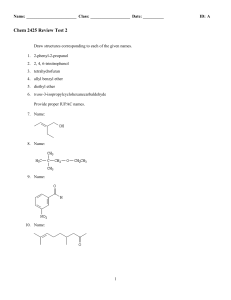
Chem 2425-Test 2 Review
... Consider the data below to answer the following question(s). Cyanohydrins are important intermediates in the synthesis of α-hydroxycarboxylic acids from ketones and aldehydes. The nitrile functional group can be hydrolyzed by aqueous acid to yield a carboxylic acid. Nitriles can also be hydrolyzed t ...
... Consider the data below to answer the following question(s). Cyanohydrins are important intermediates in the synthesis of α-hydroxycarboxylic acids from ketones and aldehydes. The nitrile functional group can be hydrolyzed by aqueous acid to yield a carboxylic acid. Nitriles can also be hydrolyzed t ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.