Chemistry of Fatty Acids
... unsaturation (Table 3). Among saturated acids, odd chain acids are lower melting than adjacent even chain acids. The presence of cis-double bonds markedly lowers the melting point, the bent chains packing less well. Trans-acids have melting points much closer to those of the corresponding saturates. ...
... unsaturation (Table 3). Among saturated acids, odd chain acids are lower melting than adjacent even chain acids. The presence of cis-double bonds markedly lowers the melting point, the bent chains packing less well. Trans-acids have melting points much closer to those of the corresponding saturates. ...
Chemistry of alcohols (powerpoint)
... Alcohols are named according to standard IUPAC rules • select the longest chain of C atoms containing the O-H group; • remove the e and add ol after the basic name • number the chain starting from the end nearer the O-H group • the number is placed after the an and before the ol ... e.g butan-2-ol • ...
... Alcohols are named according to standard IUPAC rules • select the longest chain of C atoms containing the O-H group; • remove the e and add ol after the basic name • number the chain starting from the end nearer the O-H group • the number is placed after the an and before the ol ... e.g butan-2-ol • ...
State the main methods used to prepare polymers?
... B. Have virtually identical physical properties C. Often have the same biological activities ...
... B. Have virtually identical physical properties C. Often have the same biological activities ...
Unit 2
... Hydrocarbon Derivatives are carbon compounds in which any hydrogen atom has been replaced by another atom (ex. F, P, Br, Cl, N). Organic compounds generally share some common physical and chemical properties. Most carbon compounds are _________ electrolytes or are very ________electrolytes, and tend ...
... Hydrocarbon Derivatives are carbon compounds in which any hydrogen atom has been replaced by another atom (ex. F, P, Br, Cl, N). Organic compounds generally share some common physical and chemical properties. Most carbon compounds are _________ electrolytes or are very ________electrolytes, and tend ...
Topic 22 Notes
... 2. Single and multiple covalent bonds a. Single covalent bonds (1) Where one pair of electrons is shared between two atoms. (2) Symbolized by one pair of dots or by a dash. b. Double covalent bonds (1) Where two pairs of electrons are shared between two atoms. (2) Symbolized by two pairs of dots or ...
... 2. Single and multiple covalent bonds a. Single covalent bonds (1) Where one pair of electrons is shared between two atoms. (2) Symbolized by one pair of dots or by a dash. b. Double covalent bonds (1) Where two pairs of electrons are shared between two atoms. (2) Symbolized by two pairs of dots or ...
Organometallic Methods for Forming and Cleaving Carbon
... while no ring-opening was observed with tetrahydropyran. Only highly reactive allyland benzylmagnesium halides participated in the transformation while no reaction occurred with other alkylmagnesium halides. ...
... while no ring-opening was observed with tetrahydropyran. Only highly reactive allyland benzylmagnesium halides participated in the transformation while no reaction occurred with other alkylmagnesium halides. ...
Direct organocatalytic enantioselective a-aminomethylation
... Trost and co-workers12 developed di-nuclear zinc organometallic complexes as catalyst for the direct catalytic enantioselective Mannich-type reactions between hydroxyarylketones and preformed imines. In addition, Jørgensen and co-workers have developed elegant direct asymmetric Mannich reactions inv ...
... Trost and co-workers12 developed di-nuclear zinc organometallic complexes as catalyst for the direct catalytic enantioselective Mannich-type reactions between hydroxyarylketones and preformed imines. In addition, Jørgensen and co-workers have developed elegant direct asymmetric Mannich reactions inv ...
Oxidation involving CO System ( O
... Metabolizes 1° and 2° amines; N must be attached to α-carbon; both C & N must have at least one replaceable H atom. 2° amines are metabolized by MAO if the substituent is a methyl group ...
... Metabolizes 1° and 2° amines; N must be attached to α-carbon; both C & N must have at least one replaceable H atom. 2° amines are metabolized by MAO if the substituent is a methyl group ...
Discovery and Exploitation of AZADO: The Highly Active Catalyst for
... beginning decades of the second millennium? Alcohol oxidation still suffers from a substantial issue of chemoselectivtiy; electron-rich groups often compete with alcohols. Another issue arises, particularly in a large-scale oxidation, associated with safety, environmental and economical reasons: tox ...
... beginning decades of the second millennium? Alcohol oxidation still suffers from a substantial issue of chemoselectivtiy; electron-rich groups often compete with alcohols. Another issue arises, particularly in a large-scale oxidation, associated with safety, environmental and economical reasons: tox ...
8.1 Alcohols, Phenols, and Ethers 8.2 Naming Alcohols
... Methyl Alcohol (CH3OH, methanol) Methyl alcohol is the simplest (smallest) alcohol and is commonly known as wood alcohol because it was once prepared by heating wood in the absence of air. Ethyl Alcohol (CH3CH2OH, ethanol) Ethyl alcohol is one of the oldest known pure organic compounds. Ethyl alcoho ...
... Methyl Alcohol (CH3OH, methanol) Methyl alcohol is the simplest (smallest) alcohol and is commonly known as wood alcohol because it was once prepared by heating wood in the absence of air. Ethyl Alcohol (CH3CH2OH, ethanol) Ethyl alcohol is one of the oldest known pure organic compounds. Ethyl alcoho ...
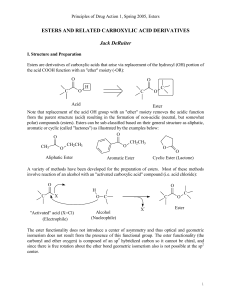
esters and related carboxylic acid derivatives
... B. Acidity: Ester carbonyl's effect on adjacent C-H bonds In the introduction of this section it was noted that esters are derivatives of carboxylic acids which arise via replacement of the -OH portion of the acid COOH function with an "ether" moiety (-OR) and that this replacement removes the acidi ...
... B. Acidity: Ester carbonyl's effect on adjacent C-H bonds In the introduction of this section it was noted that esters are derivatives of carboxylic acids which arise via replacement of the -OH portion of the acid COOH function with an "ether" moiety (-OR) and that this replacement removes the acidi ...
Oxidation
... same). Such as: HBr, HOH, HNO2, HCl, etc 10. Addition of a species Y-Y’ will definitely change the oxidation state of the reaction. Therefore, addition of Y-Y’ (eg. Br-Br) to a double bond is an Oxidation, however, elimination of Y-Y’ from a single bond is reduction. ...
... same). Such as: HBr, HOH, HNO2, HCl, etc 10. Addition of a species Y-Y’ will definitely change the oxidation state of the reaction. Therefore, addition of Y-Y’ (eg. Br-Br) to a double bond is an Oxidation, however, elimination of Y-Y’ from a single bond is reduction. ...
Chapter-16B
... Characteristic Reactions • in the general reaction, we showed the nucleophile as an anion; this need not be the case • neutral molecules such as water, alcohols, ammonia, and amines can also serve as nucleophiles • in the general reaction, we showed the leaving group as an anion to illustrate an im ...
... Characteristic Reactions • in the general reaction, we showed the nucleophile as an anion; this need not be the case • neutral molecules such as water, alcohols, ammonia, and amines can also serve as nucleophiles • in the general reaction, we showed the leaving group as an anion to illustrate an im ...
lec-3- 211( Elim+ Re..
... Oxidation is the beginning of the deterioration process. Think of how a slice of apple turns brown when exposed to air. Oxidation leads to the formation of free radicals which are unstable molecules in the body that have one unpaired electron. They can cause oxidation and damage to the cells. This ...
... Oxidation is the beginning of the deterioration process. Think of how a slice of apple turns brown when exposed to air. Oxidation leads to the formation of free radicals which are unstable molecules in the body that have one unpaired electron. They can cause oxidation and damage to the cells. This ...
Organic for Forensic Science
... ether: flash point -45 degrees C; ignition temperature 180 degrees C; explosive limits 1.9% 36% ...
... ether: flash point -45 degrees C; ignition temperature 180 degrees C; explosive limits 1.9% 36% ...
Synthesis of Natural Products and Related Compounds using Enyne
... by deprotection of the Boc group. Hetero-Michael reaction of 65 gave the isoquinoline derivative 66, which was converted into dienyne 67. Since the tertiary amine of 67 coordinates to the ruthenium catalyst and the catalytic activity is decreased, 67 was converted into 67·HCl and dienyne metathesis ...
... by deprotection of the Boc group. Hetero-Michael reaction of 65 gave the isoquinoline derivative 66, which was converted into dienyne 67. Since the tertiary amine of 67 coordinates to the ruthenium catalyst and the catalytic activity is decreased, 67 was converted into 67·HCl and dienyne metathesis ...
15alcpp - Knockhardy
... Alcohols are named according to standard IUPAC rules • select the longest chain of C atoms containing the O-H group; • remove the e and add ol after the basic name • number the chain starting from the end nearer the O-H group • the number is placed after the an and before the ol ... e.g butan-2-ol • ...
... Alcohols are named according to standard IUPAC rules • select the longest chain of C atoms containing the O-H group; • remove the e and add ol after the basic name • number the chain starting from the end nearer the O-H group • the number is placed after the an and before the ol ... e.g butan-2-ol • ...
Naming Organic Compounds
... CH3-CH2Cl + KOH CH3-CH2OH + KCl CH3-CH2OH + HCl CH3-CH2Cl + H2O CH3CH3 + Cl2 CH3CH2Cl + HCl ...
... CH3-CH2Cl + KOH CH3-CH2OH + KCl CH3-CH2OH + HCl CH3-CH2Cl + H2O CH3CH3 + Cl2 CH3CH2Cl + HCl ...
Chapter 14 - faculty at Chemeketa
... CH3COOH CH3CH2COOH CH3(CH2)2COOH CH3(CH2)3COOH CH3(CH2)4COOH CH3(CH2)5COOH CH3(CH2)6COOH ...
... CH3COOH CH3CH2COOH CH3(CH2)2COOH CH3(CH2)3COOH CH3(CH2)4COOH CH3(CH2)5COOH CH3(CH2)6COOH ...
Stereochemistry Tutorials: Assigning R/S and E/Z
... With all four priorities determined, we change the perspective to put the lowest priority group in the back (use a model!). The priorities decrease in a clockwise order so the stereocenter has the R absolute configuration. Priorities decrease clockwise HO ...
... With all four priorities determined, we change the perspective to put the lowest priority group in the back (use a model!). The priorities decrease in a clockwise order so the stereocenter has the R absolute configuration. Priorities decrease clockwise HO ...
Stereochemistry Tutorials: Assigning R/S and E/Z
... With all four priorities determined, we change the perspective to put the lowest priority group in the back (use a model!). The priorities decrease in a clockwise order so the stereocenter has the R absolute configuration. Priorities decrease clockwise HO ...
... With all four priorities determined, we change the perspective to put the lowest priority group in the back (use a model!). The priorities decrease in a clockwise order so the stereocenter has the R absolute configuration. Priorities decrease clockwise HO ...
Isomeric Product Detection in the
... products is attributed to the lack of tertiary carbons in octacosane. Zhang et al.13 showed that the heterogeneous reaction of OH with cholestane (a C27H48 solid cyclic alkane with a branched aliphatic side chain) yielded few ring-opening oxidation products. This is in contrast to gas phase reaction ...
... products is attributed to the lack of tertiary carbons in octacosane. Zhang et al.13 showed that the heterogeneous reaction of OH with cholestane (a C27H48 solid cyclic alkane with a branched aliphatic side chain) yielded few ring-opening oxidation products. This is in contrast to gas phase reaction ...
ALDEHYDES AND KETONES I. NUCLEOPHILIC ADDITION TO …
... possess carbonyl groups so that they can act as a “handle” in enzyme-substrate binding. The carbonyl group may have no other chemical purpose than just this one! WWU Chemistry ...
... possess carbonyl groups so that they can act as a “handle” in enzyme-substrate binding. The carbonyl group may have no other chemical purpose than just this one! WWU Chemistry ...
Detergents The main surfactants used in detergents and personal
... progressively replaced alkylbenzene sulfonates (ABS) for biodegradability reasons and vegetable oilbased fatty alcohol sulfates (PAS), which are biodegradable and renewable, are slowly gaining ground, gradually replacing probably LAS. The new molecules such as alkyl polyglucosides (APG), Nmethyl glu ...
... progressively replaced alkylbenzene sulfonates (ABS) for biodegradability reasons and vegetable oilbased fatty alcohol sulfates (PAS), which are biodegradable and renewable, are slowly gaining ground, gradually replacing probably LAS. The new molecules such as alkyl polyglucosides (APG), Nmethyl glu ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.