Alcohols
... In phenols the -OH group is directly attached to a carbon that is part of an aromatic ring. Alcohols and phenols are similar in some ways, but there are enough differences so that they are considered different functional groups. One major difference is that phenols are typically about a million ...
... In phenols the -OH group is directly attached to a carbon that is part of an aromatic ring. Alcohols and phenols are similar in some ways, but there are enough differences so that they are considered different functional groups. One major difference is that phenols are typically about a million ...
HIGHLIGHTS OF NUCLEOPHILIC SUBSTITUTION REACTIONS
... 4. LEAVING GROUP. The nature of the leaving group has more of an effect on the reaction rate (faster or slower) than it does on whether the reaction will follow an Sn1 or an Sn2 mechanism. The most important thing to remember in this regard is that good leaving groups are weak bases. a) All halogens ...
... 4. LEAVING GROUP. The nature of the leaving group has more of an effect on the reaction rate (faster or slower) than it does on whether the reaction will follow an Sn1 or an Sn2 mechanism. The most important thing to remember in this regard is that good leaving groups are weak bases. a) All halogens ...
Summary of Reactions Which Will Appear on Exams
... 42. REACTIONS OF GRIGNARD REAGENTS AND ORGANOLITHIUM COMPOUNDS WITH ESTERS TO FORM TERTIARY ALCOHOLS ...
... 42. REACTIONS OF GRIGNARD REAGENTS AND ORGANOLITHIUM COMPOUNDS WITH ESTERS TO FORM TERTIARY ALCOHOLS ...
Main Group Organometallic Compounds
... Highly polar, covalent – Li, Al alkyls, Grignard reagents • Polarity is a source of reactivity • Adopt associated structures – bonding power of electron pairs spread over more than 2 atoms ...
... Highly polar, covalent – Li, Al alkyls, Grignard reagents • Polarity is a source of reactivity • Adopt associated structures – bonding power of electron pairs spread over more than 2 atoms ...
Answers to exam-style questions Topic 10
... more positively charged region in a molecule (region with lower electron density) and donates a lone pair of electrons to form a covalent bond. ...
... more positively charged region in a molecule (region with lower electron density) and donates a lone pair of electrons to form a covalent bond. ...
Laboratory 21: Properties of Alkanes, Alkenes, and Alkynes
... Hydrocarbons can be divided into several different classes depending on the functional group present. The following four classes of compounds will be explored. Alkanes Are composed of hydrocarbons having only single bonds between carbon (C−C) atoms. They are often referred to as saturated hydrocarbo ...
... Hydrocarbons can be divided into several different classes depending on the functional group present. The following four classes of compounds will be explored. Alkanes Are composed of hydrocarbons having only single bonds between carbon (C−C) atoms. They are often referred to as saturated hydrocarbo ...
Oxidation of Alcohols
... • A suitable oxidising agent is a solution containing acidified dichromate ions (H+ and Cr O 2-). • These ions come from a mixture of K Cr O and sulphuric acid. • During the reaction there will be a colour change of orange to green. ...
... • A suitable oxidising agent is a solution containing acidified dichromate ions (H+ and Cr O 2-). • These ions come from a mixture of K Cr O and sulphuric acid. • During the reaction there will be a colour change of orange to green. ...
Reactions of Alcohols
... SN2 reaction between R-X and R-OWE NEED TO CONSIDER STERIC HINDERANCE. This might lead to E2! ...
... SN2 reaction between R-X and R-OWE NEED TO CONSIDER STERIC HINDERANCE. This might lead to E2! ...
CHEMISTRY 105
... red chains are both four C’s long. In the third structure, the red chain is 5 C’s long. The longest chain isn’t always left to right. It just happened that way here. But it is necessary to find the longest carbon chain first. The name of this parent chain will be pentane (3methylpentane, to give the ...
... red chains are both four C’s long. In the third structure, the red chain is 5 C’s long. The longest chain isn’t always left to right. It just happened that way here. But it is necessary to find the longest carbon chain first. The name of this parent chain will be pentane (3methylpentane, to give the ...
Dissertation:
... Compounds such as hydroxyesters: methyl and ethyl lactate, and alcohols containing chlorine and fluorine (2-chloroethanol and 2,2,2-trifluoroethanol), allyl alcohol having a carbon-carbon double bonds (C=C) and long-chain aliphatic alcohols (C10-C16) were used. It has been shown that in the presence ...
... Compounds such as hydroxyesters: methyl and ethyl lactate, and alcohols containing chlorine and fluorine (2-chloroethanol and 2,2,2-trifluoroethanol), allyl alcohol having a carbon-carbon double bonds (C=C) and long-chain aliphatic alcohols (C10-C16) were used. It has been shown that in the presence ...
Organic Chemistry - Moorpark College
... PHENOLS For a phenol, R-OH ,the R is an aryl group (based on a benzene ring, C6H5OH). The simplest phenol is phenol (C6H5OH) where the group name comes from. ETHER The general formula for an ether is R-O-R The R and R' may be the same or different. The simplest ether is dimethyl ether For complicate ...
... PHENOLS For a phenol, R-OH ,the R is an aryl group (based on a benzene ring, C6H5OH). The simplest phenol is phenol (C6H5OH) where the group name comes from. ETHER The general formula for an ether is R-O-R The R and R' may be the same or different. The simplest ether is dimethyl ether For complicate ...
5.6 Structure and properties of polymers 12.2 Alkenes 5.3 Bonds
... as the parent alkane for naming. Ethers can be thought of as being derived from water by replacing both the H atoms with alkyl groups. Ether molecules are only slightly polar and the attractive force between the molecules are relatively weak. There are no H atoms attached to the oxygen to form hydro ...
... as the parent alkane for naming. Ethers can be thought of as being derived from water by replacing both the H atoms with alkyl groups. Ether molecules are only slightly polar and the attractive force between the molecules are relatively weak. There are no H atoms attached to the oxygen to form hydro ...
Organometallic Chemistry
... • Crotyl organometallics undergo 1,3-shifts of the metal at rt. • For the stereocontrolled use of allylic organometallic reagents in synthesis, it is important that the stereoisomeric reagents not equilibrate under the reaction conditions and add to C=O regioselectively and irreversibly. • Of the va ...
... • Crotyl organometallics undergo 1,3-shifts of the metal at rt. • For the stereocontrolled use of allylic organometallic reagents in synthesis, it is important that the stereoisomeric reagents not equilibrate under the reaction conditions and add to C=O regioselectively and irreversibly. • Of the va ...
Nucleophilic
... substitution and elimination products SN2 is favored with nucleophiles that are weak bases cyanide ion, azide ion, thiolate ion, halide ion Tertiary Halides: E2 elimination occurs with strong bases such as HO–, RO–, H2N– (strongly basic conditions) E1 elimination occurs with heat and weak bases such ...
... substitution and elimination products SN2 is favored with nucleophiles that are weak bases cyanide ion, azide ion, thiolate ion, halide ion Tertiary Halides: E2 elimination occurs with strong bases such as HO–, RO–, H2N– (strongly basic conditions) E1 elimination occurs with heat and weak bases such ...
Mechanism of Dissolving Metal Reduction
... • Dihydroxylation can also be carried out by using a catalytic amount of OsO4, if the oxidant N-methylmorpholine N-oxide (NMO) is added. • In the catalytic process, dihydroxylation of the double bond converts the Os8+ oxidant into an Os6+ product, which is then re-oxidized by NMO to Os8+. ...
... • Dihydroxylation can also be carried out by using a catalytic amount of OsO4, if the oxidant N-methylmorpholine N-oxide (NMO) is added. • In the catalytic process, dihydroxylation of the double bond converts the Os8+ oxidant into an Os6+ product, which is then re-oxidized by NMO to Os8+. ...
Name Class Date 23.4 Polymers Organic compounds can bond
... Condensation Polymers When a condensation polymer is formed, water is also produced. There are two functional groups on each monomer in condensation polymerization. Polyesters and polyamides are two kinds of condensation polymers which are used to make many different kinds of products. Tough, flame ...
... Condensation Polymers When a condensation polymer is formed, water is also produced. There are two functional groups on each monomer in condensation polymerization. Polyesters and polyamides are two kinds of condensation polymers which are used to make many different kinds of products. Tough, flame ...
organic chem notes
... common methods of making nylon for fiber applications. In one approach, molecules with an acid (COOH) group on each end are reacted with molecules containing amine (NH2) groups on each end. The resulting nylon is named on the basis of the number of carbon atoms separating the two acid groups and the ...
... common methods of making nylon for fiber applications. In one approach, molecules with an acid (COOH) group on each end are reacted with molecules containing amine (NH2) groups on each end. The resulting nylon is named on the basis of the number of carbon atoms separating the two acid groups and the ...
17.2.3 Interhalogen compounds(65-67)
... the X-Fequatofiddistance but the mean X-F distance is very similar to that in the corresponding monofluoride. The structure of crystalline IC13 is quite different, being built up of planar 12Cls molecules separated by normal van der Waals' distances between the Cl atoms (Fig. 17.7~).The terminal I-C ...
... the X-Fequatofiddistance but the mean X-F distance is very similar to that in the corresponding monofluoride. The structure of crystalline IC13 is quite different, being built up of planar 12Cls molecules separated by normal van der Waals' distances between the Cl atoms (Fig. 17.7~).The terminal I-C ...
1 Chapter 8: Nucleophilic Substitution 8.1: Functional Group
... substitution and elimination products SN2 is favored with nucleophiles that are weak bases cyanide ion, azide ion, thiolate ion, halide ion Tertiary Halides: E2 elimination occurs with strong bases such as HO–, RO–, H2N– (strongly basic conditions) E1 elimination occurs with heat and weak bases such ...
... substitution and elimination products SN2 is favored with nucleophiles that are weak bases cyanide ion, azide ion, thiolate ion, halide ion Tertiary Halides: E2 elimination occurs with strong bases such as HO–, RO–, H2N– (strongly basic conditions) E1 elimination occurs with heat and weak bases such ...
Ch14 Lecture
... A tertiary (3o) alkyl halide has an X group on a C bonded only to 3 C atoms. 3o ...
... A tertiary (3o) alkyl halide has an X group on a C bonded only to 3 C atoms. 3o ...
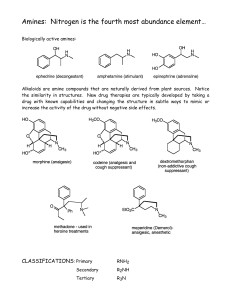
Amines: Nitrogen is the fourth most abundance element…
... We can use acid-base chemistry to separate them. Consider their characteristics: naphthalene is a neutral organic compound without any acidic nor basic functionality. Benzoic acid has an acidic proton that reacts with bases. Pyridine is a base that reacts with acids. Let’s separate them. Add aqueou ...
... We can use acid-base chemistry to separate them. Consider their characteristics: naphthalene is a neutral organic compound without any acidic nor basic functionality. Benzoic acid has an acidic proton that reacts with bases. Pyridine is a base that reacts with acids. Let’s separate them. Add aqueou ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.