Chapter 24. Amines
... • Amides (RCONH2) in general are not proton acceptors except in very strong acid • The C=O group is strongly electron-withdrawing, making the N a very weak base • Addition of a proton occurs on O but this destroys the double bond character of C=O as a requirement of stabilization by N ...
... • Amides (RCONH2) in general are not proton acceptors except in very strong acid • The C=O group is strongly electron-withdrawing, making the N a very weak base • Addition of a proton occurs on O but this destroys the double bond character of C=O as a requirement of stabilization by N ...
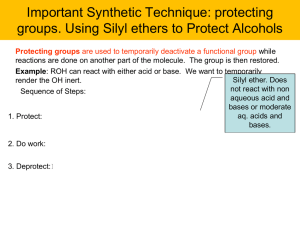
The loss of water (dehydration) and the loss of hydrogen are two
... The loss of water (dehydration) and the loss of hydrogen are two important reactions of alcohols. In the presence of heat and a strong catalyst an alcohol is dehydrated to an alkene. A water molecule splits out and a carbon-carbon double bond emerges. The pieces of the water molecule, one H and one ...
... The loss of water (dehydration) and the loss of hydrogen are two important reactions of alcohols. In the presence of heat and a strong catalyst an alcohol is dehydrated to an alkene. A water molecule splits out and a carbon-carbon double bond emerges. The pieces of the water molecule, one H and one ...
Recall
... The functionality (OH) has remained at the end of the chain. We could make it even longer by repeating the above sequence. Note attack on less hindered carbon Now a substituted oxirane… ...
... The functionality (OH) has remained at the end of the chain. We could make it even longer by repeating the above sequence. Note attack on less hindered carbon Now a substituted oxirane… ...
Document
... • Cr6+ oxidations are characterized by a color change, as the redorange Cr6+ reagent is reduced to green Cr3+. • Some devices used to measure blood alcohol content make use of this color change—Oxidation of CH3CH2OH, the 1° alcohol in alcoholic beverages, with orange K2Cr2O7 forms CH3COOH and ...
... • Cr6+ oxidations are characterized by a color change, as the redorange Cr6+ reagent is reduced to green Cr3+. • Some devices used to measure blood alcohol content make use of this color change—Oxidation of CH3CH2OH, the 1° alcohol in alcoholic beverages, with orange K2Cr2O7 forms CH3COOH and ...
CHEMISTRY 1000
... practice is to convert R-OH into R-OH2+. We saw an example of this approach in the previous section when we looked at SN1 reactions and rearrangements: ...
... practice is to convert R-OH into R-OH2+. We saw an example of this approach in the previous section when we looked at SN1 reactions and rearrangements: ...
32 GRIGNARD REACTION Alkyl halides can react with magnesium
... All your apparatus must be ABSOLUTELY DRY for this experiment, since Grignard reagent reacts with water to give hydrocarbons (benzene in this case). Mount a 50 mL round bottom flask with a condenser. Do not run water through your condenser. Place 0.7 g Mg turnings in the flask. Add 10 mL of dry ethe ...
... All your apparatus must be ABSOLUTELY DRY for this experiment, since Grignard reagent reacts with water to give hydrocarbons (benzene in this case). Mount a 50 mL round bottom flask with a condenser. Do not run water through your condenser. Place 0.7 g Mg turnings in the flask. Add 10 mL of dry ethe ...
CHM 3200 - Miami Dade College
... Survey of Organic Chemistry 3 credits Course Description: This one-semester course briefly examines the structure, synthesis, nomenclature and reactivity of selected mono- and poly-functional organic compounds. Theories that relate the structure of organic molecules to their chemical reactivity will ...
... Survey of Organic Chemistry 3 credits Course Description: This one-semester course briefly examines the structure, synthesis, nomenclature and reactivity of selected mono- and poly-functional organic compounds. Theories that relate the structure of organic molecules to their chemical reactivity will ...
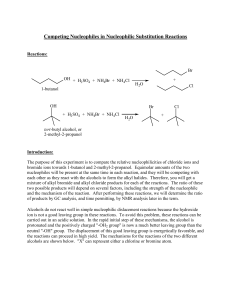
Competing Nucleophiles in Nucleophilic Substitution Reactions
... be able to calculate the actual amounts of reagents in each reaction. Note: this solventnucleophile medium contains a high concentration of sulfuric acid. Handle it with care! Using a warm 25 mL graduated cylinder, obtain 20.0 mL of the solvent-nucleophile medium. The graduated cylinder must be warm ...
... be able to calculate the actual amounts of reagents in each reaction. Note: this solventnucleophile medium contains a high concentration of sulfuric acid. Handle it with care! Using a warm 25 mL graduated cylinder, obtain 20.0 mL of the solvent-nucleophile medium. The graduated cylinder must be warm ...
Elimination Reactions
... In this, two-step process, the rate of the reaction is dependent on the rate of ionization of the substrate (as was the case in the SN1 reaction) ...
... In this, two-step process, the rate of the reaction is dependent on the rate of ionization of the substrate (as was the case in the SN1 reaction) ...
Chapter 9
... reactions, their poor leaving group must first be converted into a good leaving group by reaction with strong acids such as HBr and HI. • HBr and HI are strong acids that are also sources of good nucleophiles (Br¯ and I¯, respectively). • When ethers react with HBr or HI, both C-O bonds are cleaved ...
... reactions, their poor leaving group must first be converted into a good leaving group by reaction with strong acids such as HBr and HI. • HBr and HI are strong acids that are also sources of good nucleophiles (Br¯ and I¯, respectively). • When ethers react with HBr or HI, both C-O bonds are cleaved ...
Synthesizing Organic Compounds
... approach is to look at the available compounds and examine the transformations they can undergo. For example, what products can we obtain from short-chain alkanes and alkenes? We can then look at the possible products of these transformations and proceed further. Because there are many possible reac ...
... approach is to look at the available compounds and examine the transformations they can undergo. For example, what products can we obtain from short-chain alkanes and alkenes? We can then look at the possible products of these transformations and proceed further. Because there are many possible reac ...
Тест за III категорија, Општински натпревар по хемија, 14 март
... Nucleophilic substitution. Nucleophilic addition. Elimination. ...
... Nucleophilic substitution. Nucleophilic addition. Elimination. ...
16.18 Summary
... Dialkyl ethers are useful solvents for organic reactions, but must be used cautiously due to their tendency to form explosive hydroperoxides by air oxidation in opened bottles. ...
... Dialkyl ethers are useful solvents for organic reactions, but must be used cautiously due to their tendency to form explosive hydroperoxides by air oxidation in opened bottles. ...
Ch. 22 Hydrocarbon Compounds
... Cis-trans isomers are most commonly found in compounds with double bonds (alkenes) Cis configuration: similar groups are on the same side of the double bond Trans configuration: similar groups are on opposite sides of the double bond ...
... Cis-trans isomers are most commonly found in compounds with double bonds (alkenes) Cis configuration: similar groups are on the same side of the double bond Trans configuration: similar groups are on opposite sides of the double bond ...
Slide 1
... Two viable reaction mechanisms exist for this reaction. In the first mechanism 2-amino substituted carbonyl compound 1 and carbonyl compound 2 react in a rate-limiting step to aldol adduct 3. This intermediate loses water in an elimination reaction to unsaturated carbonyl compound 4 and then loses w ...
... Two viable reaction mechanisms exist for this reaction. In the first mechanism 2-amino substituted carbonyl compound 1 and carbonyl compound 2 react in a rate-limiting step to aldol adduct 3. This intermediate loses water in an elimination reaction to unsaturated carbonyl compound 4 and then loses w ...
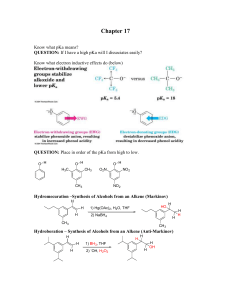
Chapter 17 - Ellis Benjamin
... Chapter 17 Know what pKa means? QUESTION: If I have a high pKa will I dissociates easily? Know what electron inductive effects do (below) ...
... Chapter 17 Know what pKa means? QUESTION: If I have a high pKa will I dissociates easily? Know what electron inductive effects do (below) ...
Brominations and Alkene Synthesis CHM 233 Review
... • ANTI addition, bromines add to opposite sides of the C=C double bond (top and bottom) • note new type of structure for indicating stereochemistry on rings, trans- in this case • NOTE, because of bromonium ion intermediate (no "free" carbocation) there are no rearrangements - we will use this infor ...
... • ANTI addition, bromines add to opposite sides of the C=C double bond (top and bottom) • note new type of structure for indicating stereochemistry on rings, trans- in this case • NOTE, because of bromonium ion intermediate (no "free" carbocation) there are no rearrangements - we will use this infor ...
CHM 331 : General Organic Chemistry
... • ANTI addition, bromines add to opposite sides of the C=C double bond (top and bottom) • note new type of structure for indicating stereochemistry on rings, trans- in this case • NOTE, because of bromonium ion intermediate (no "free" carbocation) there are no rearrangements - we will use this infor ...
... • ANTI addition, bromines add to opposite sides of the C=C double bond (top and bottom) • note new type of structure for indicating stereochemistry on rings, trans- in this case • NOTE, because of bromonium ion intermediate (no "free" carbocation) there are no rearrangements - we will use this infor ...
Alcohols, Phenols and Ethers
... 3. It is soluble in water but insoluble in ether. 4. Due to excessive H-bonding, it is highly viscous and has high boiling point. Chemical Properties It gives all the general reactions given by -OR group but 2° OR is less reactive as compared to 1° ...
... 3. It is soluble in water but insoluble in ether. 4. Due to excessive H-bonding, it is highly viscous and has high boiling point. Chemical Properties It gives all the general reactions given by -OR group but 2° OR is less reactive as compared to 1° ...
Microsoft Word - Final Exam Study Guide
... B. Would you expect the elimination of cis-1-t-butyl-4-chlorocyclohexane using a strong base to be faster or slower than the elimination of chlorocyclohexane with a strong base? ...
... B. Would you expect the elimination of cis-1-t-butyl-4-chlorocyclohexane using a strong base to be faster or slower than the elimination of chlorocyclohexane with a strong base? ...
Outline
... red chains are both four C’s long. In the third structure, the red chain is 5 C’s long. The longest chain isn’t always left to right. It just happened that way here. But it is necessary to find the longest carbon chain first. The name of this parent chain will be pentane (3methylpentane, to give the ...
... red chains are both four C’s long. In the third structure, the red chain is 5 C’s long. The longest chain isn’t always left to right. It just happened that way here. But it is necessary to find the longest carbon chain first. The name of this parent chain will be pentane (3methylpentane, to give the ...
Carboxylic Acid Derivatives and Nitriles
... Like amides, nitriles are reduced in LiAlH4 to primary amines. The reaction is quite clean, usually affording the amine in >80% yield. However, because nitriles are often made from amides, it is often easier to convert the amide to the amine, rather than go through the nitrile. The only real excepti ...
... Like amides, nitriles are reduced in LiAlH4 to primary amines. The reaction is quite clean, usually affording the amine in >80% yield. However, because nitriles are often made from amides, it is often easier to convert the amide to the amine, rather than go through the nitrile. The only real excepti ...
Lecture 2 - UCLA Chemistry and Biochemistry
... Example: Elimination from Cyclohexanol The equilibrium constant for many elimination reactions is low because neither the enthalpy (DH=23.9 kJ, ) nor the entropy (DS=84.91 J, ) changes much and they also display opposing trends. Thus, the equilibrium constant is Keq=1.8 at 25 oC and Keq=8 at 80 oC ...
... Example: Elimination from Cyclohexanol The equilibrium constant for many elimination reactions is low because neither the enthalpy (DH=23.9 kJ, ) nor the entropy (DS=84.91 J, ) changes much and they also display opposing trends. Thus, the equilibrium constant is Keq=1.8 at 25 oC and Keq=8 at 80 oC ...
Chapter: Haloalkanes and Haloarenes
... new forces of attraction set up between haloalkanes and the solvent molecules are of same strength as the forces of attraction being broken. (ii) A mixture of equal amounts of two enantiomers is known as racemic mixture. For example: When a 3o halide undergoes substitution with KOH, the reaction pro ...
... new forces of attraction set up between haloalkanes and the solvent molecules are of same strength as the forces of attraction being broken. (ii) A mixture of equal amounts of two enantiomers is known as racemic mixture. For example: When a 3o halide undergoes substitution with KOH, the reaction pro ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.