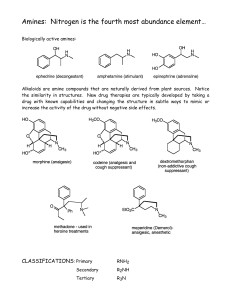
Amines: Nitrogen is the fourth most abundance element…
... We can use acid-base chemistry to separate them. Consider their characteristics: naphthalene is a neutral organic compound without any acidic nor basic functionality. Benzoic acid has an acidic proton that reacts with bases. Pyridine is a base that reacts with acids. Let’s separate them. Add aqueou ...
... We can use acid-base chemistry to separate them. Consider their characteristics: naphthalene is a neutral organic compound without any acidic nor basic functionality. Benzoic acid has an acidic proton that reacts with bases. Pyridine is a base that reacts with acids. Let’s separate them. Add aqueou ...
PowerPoint Presentation - Chapter 1
... Select the major organic product when (S)-2propanol is reacted with SOCl2 in pyridine followed by the addition of NaSH in ethanol. A) ...
... Select the major organic product when (S)-2propanol is reacted with SOCl2 in pyridine followed by the addition of NaSH in ethanol. A) ...
revised hydrocarbons alkenes cycloalkenes
... combines with anionic conjugate base. Reaction progress can be studied by the given energy diagram for addition of hydrogen chloride to propane. The carbocation intermediate formed in the first step of addition reaction directly influences the activation energy for this step. A more stable carbocati ...
... combines with anionic conjugate base. Reaction progress can be studied by the given energy diagram for addition of hydrogen chloride to propane. The carbocation intermediate formed in the first step of addition reaction directly influences the activation energy for this step. A more stable carbocati ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... c) Why oxymercuration-demercuration method of preparation of alcohols follows anti addition. ...
... c) Why oxymercuration-demercuration method of preparation of alcohols follows anti addition. ...
Nuggets of Knowledge for Chapter 13 – Alcohols (II)
... OH is not a good leaving group, so nucleophiles cannot attack them. o Reduction: Although in theory the alcohol could lose a bond to oxygen, there are no reagents available to accomplish this reaction (the only way to do this is using more than one step). ...
... OH is not a good leaving group, so nucleophiles cannot attack them. o Reduction: Although in theory the alcohol could lose a bond to oxygen, there are no reagents available to accomplish this reaction (the only way to do this is using more than one step). ...
Solution-Phase Combinatorial Chemistry
... focus was on solid-phase approaches due to the many advantages. • Solution chemistry was not regarded as being suitable for combinatorial chemistry because of the often tedious isolation and purification. • It was first used for easily synthesized compound classes [amides, sulfonamides, ureas, heter ...
... focus was on solid-phase approaches due to the many advantages. • Solution chemistry was not regarded as being suitable for combinatorial chemistry because of the often tedious isolation and purification. • It was first used for easily synthesized compound classes [amides, sulfonamides, ureas, heter ...
... from tertiary alcohols 1 and alkenes 2 via a palladium-catalyzed hydroxyl-directed C–H olefination– intramolecular oxidative cyclization sequence is described. Optimization of the reaction conditions showed that the amino acid ligand shown is critical for high conversion. Instructively, hexafluorobe ...
organic synthesis
... the nucleophile can attack from above and below there is an equal chance of each possibility a mixture of optically active isomers is produced only occurs if different groups are attached to the carbonyl group ...
... the nucleophile can attack from above and below there is an equal chance of each possibility a mixture of optically active isomers is produced only occurs if different groups are attached to the carbonyl group ...
AP Ch. 25 Notes
... • Amines are organic bases where R may be H or a hydrocarbon group. • Just as alcohols, (R – OH), can be thought of organic forms of water, (H – OH) amines can be thought of organic forms of ammonia, NH3. Example: ethylamine, (CH3CH2NH2) • Amines are among the most noxious-smelling of all organic ...
... • Amines are organic bases where R may be H or a hydrocarbon group. • Just as alcohols, (R – OH), can be thought of organic forms of water, (H – OH) amines can be thought of organic forms of ammonia, NH3. Example: ethylamine, (CH3CH2NH2) • Amines are among the most noxious-smelling of all organic ...
Alcohols from Alkenes: Oxymercuration–Demercuration
... Mechanism of Oxymercuration–Demercuration ...
... Mechanism of Oxymercuration–Demercuration ...
RULE
... Acid conjugate base (HSO4- counterion) - the concentration of this species is much less than that of H2O (remember 55 M!), so a carbocation is much more likely to encounter water in this fast step 2 than the counterion. Why not consider using -OH as our Nu:? We could simplify things by eliminating o ...
... Acid conjugate base (HSO4- counterion) - the concentration of this species is much less than that of H2O (remember 55 M!), so a carbocation is much more likely to encounter water in this fast step 2 than the counterion. Why not consider using -OH as our Nu:? We could simplify things by eliminating o ...
Experiment 7 — Nucleophilic Substitution
... ethanol for SN1 reactions. Briefly explain why (a) the SN1 reaction pathway is disfavored with NaI/acetone, and (b) why the SN2 pathway is disfavored with AgNO3/EtOH. Nucleophilic substitution is one of the most useful and well studied class of organic reactions. These reactions can occur by a range ...
... ethanol for SN1 reactions. Briefly explain why (a) the SN1 reaction pathway is disfavored with NaI/acetone, and (b) why the SN2 pathway is disfavored with AgNO3/EtOH. Nucleophilic substitution is one of the most useful and well studied class of organic reactions. These reactions can occur by a range ...
16.2: Structure and Bonding in Ethers and Epoxides
... The ether oxygen is sp3-hybridized and tetrahedral. In general, the C-O bonds of ethers have low reactivity. 16.3: Physical Properties of Ethers The O-H group of alcohols act as both an H-bond donor (Lewis acid) and H-bond acceptor (Lewis base). Ethers are only H-bond acceptors (Lewis base) 16.4: Cr ...
... The ether oxygen is sp3-hybridized and tetrahedral. In general, the C-O bonds of ethers have low reactivity. 16.3: Physical Properties of Ethers The O-H group of alcohols act as both an H-bond donor (Lewis acid) and H-bond acceptor (Lewis base). Ethers are only H-bond acceptors (Lewis base) 16.4: Cr ...
esters - wellswaysciences
... • Artificial flavourings are made by mixing synthetic esters but they are only ever approximate because it would be too expensive to include all the flavour compounds present in real fruit etc. ...
... • Artificial flavourings are made by mixing synthetic esters but they are only ever approximate because it would be too expensive to include all the flavour compounds present in real fruit etc. ...
Functional Groups
... of compounds and their Name of functional group characteristic functional groups: ...
... of compounds and their Name of functional group characteristic functional groups: ...
Grant MacEwan College - Faculty Web Pages
... Description: This is the second course in organic chemistry. The topics covered include structural and chemical properties of alkenes, alkynes, alcohols, phenols, ethers, aromatic compounds. Aldehyde, ketones, amines, carboxylic acids, and carboxylic acid derivatives. Illustration of these functiona ...
... Description: This is the second course in organic chemistry. The topics covered include structural and chemical properties of alkenes, alkynes, alcohols, phenols, ethers, aromatic compounds. Aldehyde, ketones, amines, carboxylic acids, and carboxylic acid derivatives. Illustration of these functiona ...
N-METAL COMPOUNDS
... incipient carbocations). Alkyl and hydride shifts then bear analogy to carbocation rearrangements. This may be an oversimplification but it makes the chemistry easier to follow. ...
... incipient carbocations). Alkyl and hydride shifts then bear analogy to carbocation rearrangements. This may be an oversimplification but it makes the chemistry easier to follow. ...
Alcohols
... attached to the same carbon as -OH • Distinguished by Lucas reagent (ZnCl2 in conc HCl) – no visible reaction: primary alcohol – solution turns cloudy in 3-5 minutes: secondary alcohol – solution turns cloudy immediately, and/or phases separate: tertiary or benzyl alcohol ...
... attached to the same carbon as -OH • Distinguished by Lucas reagent (ZnCl2 in conc HCl) – no visible reaction: primary alcohol – solution turns cloudy in 3-5 minutes: secondary alcohol – solution turns cloudy immediately, and/or phases separate: tertiary or benzyl alcohol ...
Alkenes Group
... • Because they have a double bond, they are called unsaturated. (Alkanes have no double bonds, so are saturated) • They are much more reactive than alkanes C2H4 + H2 ---C3H6 The hydrogen just add on, so this is called an addition reaction • Alkenes also do an addition reaction with steam, to form c ...
... • Because they have a double bond, they are called unsaturated. (Alkanes have no double bonds, so are saturated) • They are much more reactive than alkanes C2H4 + H2 ---C3H6 The hydrogen just add on, so this is called an addition reaction • Alkenes also do an addition reaction with steam, to form c ...
Alkenes: Overview
... •Liquid (solid if < 6° C) was condensing in the gas lines and blocking the flow. •Michael Faraday isolated the unknown compound and tried to characterise it. •Combustion gave C6H6 - Benzene. •Put yourself in Faraday’s place. •C6H6 suggests unsaturation. (Alkanes are normally CnH2n+2) •Logically the ...
... •Liquid (solid if < 6° C) was condensing in the gas lines and blocking the flow. •Michael Faraday isolated the unknown compound and tried to characterise it. •Combustion gave C6H6 - Benzene. •Put yourself in Faraday’s place. •C6H6 suggests unsaturation. (Alkanes are normally CnH2n+2) •Logically the ...
Dehydrating Cyclohexanol
... Br2 can react with carbon-carbon double bonds and form some colorless product which is different from the red-brown color of Br2. ...
... Br2 can react with carbon-carbon double bonds and form some colorless product which is different from the red-brown color of Br2. ...
Chapter 24. Amines
... • Amides (RCONH2) in general are not proton acceptors except in very strong acid • The C=O group is strongly electron-withdrawing, making the N a very weak base • Addition of a proton occurs on O but this destroys the double bond character of C=O as a requirement of stabilization by N ...
... • Amides (RCONH2) in general are not proton acceptors except in very strong acid • The C=O group is strongly electron-withdrawing, making the N a very weak base • Addition of a proton occurs on O but this destroys the double bond character of C=O as a requirement of stabilization by N ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.























