Paper
... (b) Name the scientist, shown in the photograph, who identified cathode rays as subatomic particles. (c) Give two differences between a nuclear reaction and a chemical reaction. (d) Calculate the percentage carbon, by mass, in methylbenzene. (e) What is (i) the conjugate acid and (ii) the conjugate ...
... (b) Name the scientist, shown in the photograph, who identified cathode rays as subatomic particles. (c) Give two differences between a nuclear reaction and a chemical reaction. (d) Calculate the percentage carbon, by mass, in methylbenzene. (e) What is (i) the conjugate acid and (ii) the conjugate ...
Bonds - MCAT Cooperative
... reduced to alcohols w strong reducing agents such as NaBH4 and LiAlH4 ...
... reduced to alcohols w strong reducing agents such as NaBH4 and LiAlH4 ...
2.10 Reactions of alcohols
... 2.10 Reactions of alcohols c. describe the following chemistry of alcohols: i. combustion ii. reaction with sodium iii. substitution reactions to form halogenoalkanes, including reaction with PCl5 and its use as a qualitative test for the presence of the –OH group iv. oxidation using potassium dichr ...
... 2.10 Reactions of alcohols c. describe the following chemistry of alcohols: i. combustion ii. reaction with sodium iii. substitution reactions to form halogenoalkanes, including reaction with PCl5 and its use as a qualitative test for the presence of the –OH group iv. oxidation using potassium dichr ...
9.1-10.5 Organic Chemistry
... compounds of carbon, called ORGANIC compounds*. *Carbon compounds that are exceptions and considered INORGANIC are compounds like: Oxides carbon monoxide (CO(g) ) and carbon dioxide (CO2(g) ), and Ionic compounds of carbon-based ions, such as carbonate CO32-, cyanide CN-, and carbide ions, SiC ( ...
... compounds of carbon, called ORGANIC compounds*. *Carbon compounds that are exceptions and considered INORGANIC are compounds like: Oxides carbon monoxide (CO(g) ) and carbon dioxide (CO2(g) ), and Ionic compounds of carbon-based ions, such as carbonate CO32-, cyanide CN-, and carbide ions, SiC ( ...
Lecture 13a - University of California, Los Angeles
... • The lone pair on the carbon forms a s-bond with a suitable d-orbital of the metal • The metal can form a p-back bond via the p*-orbital of the CO ligand • Electron-rich metals i.e., late transition metals in low oxidation states are more likely to donate electrons for the back bonding • A strong p ...
... • The lone pair on the carbon forms a s-bond with a suitable d-orbital of the metal • The metal can form a p-back bond via the p*-orbital of the CO ligand • Electron-rich metals i.e., late transition metals in low oxidation states are more likely to donate electrons for the back bonding • A strong p ...
... Green Technology has been mentioned in the past few years due to the environmental problems are still increasing as a result of the environmental care products demand increased. The biological processes have thus been developed for any manufacturing steps. Alkyl glycosides are classified as a non-io ...
10. Alkyl Halides
... mechanism. The rxn rate = k x [RX][Base] The essential feature of the E2 mechanism is that it is a one step process without intermediate. As the attacking base / nucleophile begins to abstract a proton from a carbon next to the leaving group, the C-H begins to break, a new carbon-carbon pi bond be ...
... mechanism. The rxn rate = k x [RX][Base] The essential feature of the E2 mechanism is that it is a one step process without intermediate. As the attacking base / nucleophile begins to abstract a proton from a carbon next to the leaving group, the C-H begins to break, a new carbon-carbon pi bond be ...
Reactions of Alkenes and Alkynes
... Bromonium ion shields one side of molecule so that reaction with Br- ion occurs only from opposite side ...
... Bromonium ion shields one side of molecule so that reaction with Br- ion occurs only from opposite side ...
Chapter 16: Ethers, Epoxides, and Sulfides
... In general, the C-O bonds of ethers have low reactivity. 16.3: Physical Properties of Ethers the O-H group of alcohols act as both an H-bond donor (Lewis acid) and H-bond acceptor (Lewis base). Ethers are only H-bond acceptors (Lewis base) 16.4: Crown Ethers (Please read) ...
... In general, the C-O bonds of ethers have low reactivity. 16.3: Physical Properties of Ethers the O-H group of alcohols act as both an H-bond donor (Lewis acid) and H-bond acceptor (Lewis base). Ethers are only H-bond acceptors (Lewis base) 16.4: Crown Ethers (Please read) ...
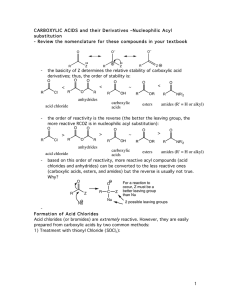
carboxylic acids esters amides (R
... (carboxylic acids, esters, and amides) but the reverse is usually not true. Why? O R ...
... (carboxylic acids, esters, and amides) but the reverse is usually not true. Why? O R ...
Double Bond
... Hydrogen gas and an alkene will react when mixed in the presence of a catalyst such as platinum or palladium. Two hydrogen atoms are added to the alkene in a reaction called hydrogenation, which is very exothermic. The heat released is called the “heat of hydrogenation” and has a typical value of ab ...
... Hydrogen gas and an alkene will react when mixed in the presence of a catalyst such as platinum or palladium. Two hydrogen atoms are added to the alkene in a reaction called hydrogenation, which is very exothermic. The heat released is called the “heat of hydrogenation” and has a typical value of ab ...
PPT CH 11
... • An alkene can be combined with a hydrogen halide such as HBr or HCl • The reaction product is an alkyl halide • Markovnikov’s Rule is followed in this reaction ...
... • An alkene can be combined with a hydrogen halide such as HBr or HCl • The reaction product is an alkyl halide • Markovnikov’s Rule is followed in this reaction ...
Get Notes - Mindset Learn
... Structural formula of the organic product formed in the test tube containing compound E. ...
... Structural formula of the organic product formed in the test tube containing compound E. ...
functional group
... Functional groups and nomenclature Role of intermolecular forces on properties Synthesis of alkyl halides from alcohols Two reaction mechanisms: SN1 and SN2 cation stability and the Hammond Postulate 5) Additional ways to prepare RX from ROH 6) Halogenation of alkanes: reactivityselectivity principl ...
... Functional groups and nomenclature Role of intermolecular forces on properties Synthesis of alkyl halides from alcohols Two reaction mechanisms: SN1 and SN2 cation stability and the Hammond Postulate 5) Additional ways to prepare RX from ROH 6) Halogenation of alkanes: reactivityselectivity principl ...
... 1 via an intramolecular palladium-catalyzed C–H activation/C–O cyclization sequence. Both EDGs and EWGs are tolerated at various positions around the ring. Yields are excellent (>75%) in almost all cases, except for R3 = CO2Et (50%) and R3 = H (42%). The stability of halogen substituents (R1 = Cl, B ...
Survey on Conditions Catalysis of Chemical Reactions
... which is normally positive, results in a high reactivity, especially with atoms that can accept electrons (be reduced), allowing the hydrogen to become positive again (normal oxidation state of +1). Precautions: It reacts with other positive centers as well, especially any slightly acidic hydrogens, ...
... which is normally positive, results in a high reactivity, especially with atoms that can accept electrons (be reduced), allowing the hydrogen to become positive again (normal oxidation state of +1). Precautions: It reacts with other positive centers as well, especially any slightly acidic hydrogens, ...
Winter 2004 Final Exam
... 1. Give a brief description and provide a suitable example of each of the following: a) Markovnikov’s rule ...
... 1. Give a brief description and provide a suitable example of each of the following: a) Markovnikov’s rule ...
CfE Higher Chemistry Homework Unit 2: Natures Chemistry
... Draw another molecule of 2-phenylethanol and use a dotted line to show where a hydrogen bond exists between the two molecules The end note of a perfume has a long lasting odour which stays with the user. An example of an end note compound is civetone. ...
... Draw another molecule of 2-phenylethanol and use a dotted line to show where a hydrogen bond exists between the two molecules The end note of a perfume has a long lasting odour which stays with the user. An example of an end note compound is civetone. ...
Chapter 21: Carboxylic Acid Derivatives
... however this reaction is easy since the leaving group Cl- is a weaker base than NH2300 ...
... however this reaction is easy since the leaving group Cl- is a weaker base than NH2300 ...
Chapter #2 - FIU Faculty Websites
... because this is the site of most chemical reactivity of a molecule The functional group is also responsible for many of the physical properties of a molecule ...
... because this is the site of most chemical reactivity of a molecule The functional group is also responsible for many of the physical properties of a molecule ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.