10 Haloalkanes and Haloarenes
... This is because of +I effect of alkyl group(s) attached to the - carbon atom of an alcohol, which facilitates the cleavage of C O bond of an alcohol and increases the reactivity of alcohol. ...
... This is because of +I effect of alkyl group(s) attached to the - carbon atom of an alcohol, which facilitates the cleavage of C O bond of an alcohol and increases the reactivity of alcohol. ...
Learning Guide for Chapter 9 - Alkyl Halides I
... What is synthesis? figuring out how to make a compound each reaction you learn will be a tool in your toolbox to make a specific change substitution - alkyl halide to other functional groups What steps should you go through? 1) look at the compound to decide what nucleophiles you could use 2) decide ...
... What is synthesis? figuring out how to make a compound each reaction you learn will be a tool in your toolbox to make a specific change substitution - alkyl halide to other functional groups What steps should you go through? 1) look at the compound to decide what nucleophiles you could use 2) decide ...
Organic Compound - TangHua2012-2013
... bond to a carbon atom. The general formula for a simple alcohol containing no rings is CnH(2n+1)OH. *Classification: Three major subsets of alcohols- 'primary' (1°), 'secondary' (2°) and 'tertiary' (3°), based upon the number of carbons the C-OH carbon is bonded to. A primary (1°) alcohol is one in ...
... bond to a carbon atom. The general formula for a simple alcohol containing no rings is CnH(2n+1)OH. *Classification: Three major subsets of alcohols- 'primary' (1°), 'secondary' (2°) and 'tertiary' (3°), based upon the number of carbons the C-OH carbon is bonded to. A primary (1°) alcohol is one in ...
Reactions of Alcohols
... -OH, to promote the reaction. • The chloride product is insoluble. • Lucas test: ZnCl2 in conc. HCl 1° alcohols react slowly or not at all. 2 alcohols react in 1-5 minutes. 3 alcohols react in less than 1 minute. ...
... -OH, to promote the reaction. • The chloride product is insoluble. • Lucas test: ZnCl2 in conc. HCl 1° alcohols react slowly or not at all. 2 alcohols react in 1-5 minutes. 3 alcohols react in less than 1 minute. ...
Naming of Aromatic Compounds
... Sources of Alkanes • Natural gas – 90 to 95 percent methane – 5 to 10 percent ethane, and – a mixture of other low-boiling alkanes, chiefly propane, butane, and 2methylpropane. • Petroleum – A thick liquid mixture of thousands of compounds, most of them hydrocarbons formed from the decomposition of ...
... Sources of Alkanes • Natural gas – 90 to 95 percent methane – 5 to 10 percent ethane, and – a mixture of other low-boiling alkanes, chiefly propane, butane, and 2methylpropane. • Petroleum – A thick liquid mixture of thousands of compounds, most of them hydrocarbons formed from the decomposition of ...
More reactions of alkenes Objective
... 3) You get a positiviely charged carbocation intermediate. The Br- now zooms over…… ...
... 3) You get a positiviely charged carbocation intermediate. The Br- now zooms over…… ...
Hein and Arena
... of reactions involved in making compounds that can be used in a separate process to manufacture an energy-rich compound called ATP. A reaction early in the citric acid cycle involves the oxidation of an alcohol. Of the two reactants shown below (each is a reactant somewhere in the cycle), which can ...
... of reactions involved in making compounds that can be used in a separate process to manufacture an energy-rich compound called ATP. A reaction early in the citric acid cycle involves the oxidation of an alcohol. Of the two reactants shown below (each is a reactant somewhere in the cycle), which can ...
Ch-1-Alkanes and isomerism-corr
... Alkanes are non- polar so are immiscible with water , they are soluble in most organic solvents. ...
... Alkanes are non- polar so are immiscible with water , they are soluble in most organic solvents. ...
TV RajanBabu Chemistry, 730 Autumn 1997
... Carbanions stabilized by other functional groups Malontes, acetoacetates, nitrocompounds etc. Enolates - kinetic vs thermodynamic - regiochemistry in unsymmetrical ketone enolates - how to prepare regiochemically pure enolates Other carbanions in synthesis - dithianes and corresponding sulfoxides, n ...
... Carbanions stabilized by other functional groups Malontes, acetoacetates, nitrocompounds etc. Enolates - kinetic vs thermodynamic - regiochemistry in unsymmetrical ketone enolates - how to prepare regiochemically pure enolates Other carbanions in synthesis - dithianes and corresponding sulfoxides, n ...
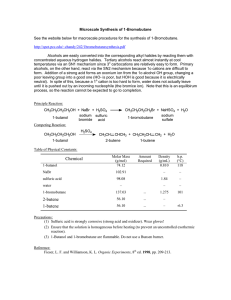
Synthesis of 1
... http://spot.pcc.edu/~chandy/242/1bromobutanesynthesis.pdf Alcohols are easily converted into the corresponding alkyl halides by reacting them with concentrated aqueous hydrogen halides. Tertiary alcohols react almost instantly at cool temperatures via an SN1 mechanism since 3o carbocations are relat ...
... http://spot.pcc.edu/~chandy/242/1bromobutanesynthesis.pdf Alcohols are easily converted into the corresponding alkyl halides by reacting them with concentrated aqueous hydrogen halides. Tertiary alcohols react almost instantly at cool temperatures via an SN1 mechanism since 3o carbocations are relat ...
F017006 - Fluorous Technologies
... F-PMB-OH is the fluorous equivalent of p-methoxybenzyl alcohol (PMB-OH) used in protecting alcohols in multi-step organic synthesis. Protection of an alcohol with F-PMB-OH and deprotection are achieved under traditional reaction conditions, with the advantage that products containing the F-PMB group ...
... F-PMB-OH is the fluorous equivalent of p-methoxybenzyl alcohol (PMB-OH) used in protecting alcohols in multi-step organic synthesis. Protection of an alcohol with F-PMB-OH and deprotection are achieved under traditional reaction conditions, with the advantage that products containing the F-PMB group ...
15 - MSU Chemistry
... The starting material for the second reaction is also achiral as it too has a plane of symmetry. The stereochemistry merely shows that the two OTs groups are on the same side of th ...
... The starting material for the second reaction is also achiral as it too has a plane of symmetry. The stereochemistry merely shows that the two OTs groups are on the same side of th ...
IUPAC nomenclature for organic chemistry
... carbon atoms of the double bond • Number the root chain from the end nearest a double bond carbon atom (or triple bond carbon atom) • The smaller of the two numbers designating the carbon atoms of the double/triple bond is used as the locator of alkenes/alkynes ...
... carbon atoms of the double bond • Number the root chain from the end nearest a double bond carbon atom (or triple bond carbon atom) • The smaller of the two numbers designating the carbon atoms of the double/triple bond is used as the locator of alkenes/alkynes ...
Grant MacEwan College - Faculty Web Pages
... Description: This is the second course in organic chemistry. The topics covered include structural and chemical properties of alkenes, alkynes, alcohols, phenols, ethers, aromatic compounds. Aldehyde, ketones, amines, carboxylic acids, and carboxylic acid derivatives. Illustration of these functiona ...
... Description: This is the second course in organic chemistry. The topics covered include structural and chemical properties of alkenes, alkynes, alcohols, phenols, ethers, aromatic compounds. Aldehyde, ketones, amines, carboxylic acids, and carboxylic acid derivatives. Illustration of these functiona ...
Carbonyl Compounds
... The reaction is reversible. It is driven to completion by using a small excess of the carboxylic acid and by distilling out the water from the reaction mixture as it is formed. ...
... The reaction is reversible. It is driven to completion by using a small excess of the carboxylic acid and by distilling out the water from the reaction mixture as it is formed. ...
Homework Packet - Chemistry from AZ
... example: ethane + oxygen water + carbon dioxide D. substitution: replacing one atom with another; saturated hydrocarbons remain saturated; includes hydrogenation or halogenation example: ethane + bromine bromoethane + hydrogen bromide E. addition: adding two atoms to either side of a double bond ...
... example: ethane + oxygen water + carbon dioxide D. substitution: replacing one atom with another; saturated hydrocarbons remain saturated; includes hydrogenation or halogenation example: ethane + bromine bromoethane + hydrogen bromide E. addition: adding two atoms to either side of a double bond ...
MHS Student Guide to Organic Chemistry
... Chemicals compounds that contain the element Carbon are known as organic compounds. “Organic” comes from the fact that until the mid 1800’s it was thought that these chemicals could only be derived from living plant or animal components. In 1828 Friedrich Woher converted the inorganic ammonium salt ...
... Chemicals compounds that contain the element Carbon are known as organic compounds. “Organic” comes from the fact that until the mid 1800’s it was thought that these chemicals could only be derived from living plant or animal components. In 1828 Friedrich Woher converted the inorganic ammonium salt ...
06 MC /08 MC /08 NMR
... Counting this cover sheet, there are a total of 9 pages- check to ensure you have all 9. All 9 pages must be turned in for grading. Use the backside of preceding pages for scratch paper. Any cheating will result in the dismissal from c/ass with an "F" grade. Please put your name or initial on each p ...
... Counting this cover sheet, there are a total of 9 pages- check to ensure you have all 9. All 9 pages must be turned in for grading. Use the backside of preceding pages for scratch paper. Any cheating will result in the dismissal from c/ass with an "F" grade. Please put your name or initial on each p ...
T. V. RajanBabu Chemistry, 730 Autumn 1997
... Michael reaction - stabilized enolates- malonates, acetoacetates, nitronates Michael reaction of kinetic enolates -special acceptors ...
... Michael reaction - stabilized enolates- malonates, acetoacetates, nitronates Michael reaction of kinetic enolates -special acceptors ...
730-2005 topics
... Carbanions stabilized by other functional groups Malontes, acetoacetates, nitrocompounds etc. Enolates - kinetic vs thermodynamic - regiochemistry in unsymmetrical ketone enolates - how to prepare regiochemically pure enolates Other carbanions in synthesis - dithianes and corresponding sulfoxides, n ...
... Carbanions stabilized by other functional groups Malontes, acetoacetates, nitrocompounds etc. Enolates - kinetic vs thermodynamic - regiochemistry in unsymmetrical ketone enolates - how to prepare regiochemically pure enolates Other carbanions in synthesis - dithianes and corresponding sulfoxides, n ...
Course No - Chemistry
... A. Inorganic Chemistry Qualitative analysis (macro) of inorganic mixture containing two cations and two anions, excluding insoluble and interfering radicals. B. Organic Chemistry : Calibration of Thermometer, determination of melting points, boiling points. Simple distillation of organic solvent-wat ...
... A. Inorganic Chemistry Qualitative analysis (macro) of inorganic mixture containing two cations and two anions, excluding insoluble and interfering radicals. B. Organic Chemistry : Calibration of Thermometer, determination of melting points, boiling points. Simple distillation of organic solvent-wat ...
Octenes from E1 versus E2 Eliminations
... Fill a 10 x 100 mm reaction tube to the 0.5 mL mark with 1-octanol (n-octyl alcohol) and insert a 1/2-inch stir bar. Add 5 drops of conc. sulfuric acid. While stirring, heat the reaction for 20 to 30 minutes. At first you will see water droplets and a cloudy liquid condensing on the walls of the rea ...
... Fill a 10 x 100 mm reaction tube to the 0.5 mL mark with 1-octanol (n-octyl alcohol) and insert a 1/2-inch stir bar. Add 5 drops of conc. sulfuric acid. While stirring, heat the reaction for 20 to 30 minutes. At first you will see water droplets and a cloudy liquid condensing on the walls of the rea ...
Key to Exam 3
... Hints: A-B, and E-J have stereochemical issues; in some cases you have to evaluate which is the least hindered side of the molecules, in other cases cis versus trans etc. Pay attention to regiochemistry—most stable carbocation. E and F are rearranged addition products. The bonds that interact best w ...
... Hints: A-B, and E-J have stereochemical issues; in some cases you have to evaluate which is the least hindered side of the molecules, in other cases cis versus trans etc. Pay attention to regiochemistry—most stable carbocation. E and F are rearranged addition products. The bonds that interact best w ...
Alkynes - IIT Portal.com
... acetylene by proton transfer from compounds that contain hydroxyl groups. Amide ion is a much stronger base than acetylide ion and converts acetylene to its conjugate base quantitatively. ...
... acetylene by proton transfer from compounds that contain hydroxyl groups. Amide ion is a much stronger base than acetylide ion and converts acetylene to its conjugate base quantitatively. ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.