Chapter 3 Molecules Molecules, Compounds, and Chemical
... found in compounds, ionic and covalent. Ionic bonds result when electrons have been transferred between atoms, resulting in oppositely charged ions that attract each other. generally found when metal atoms bonded to nonmetal atoms ...
... found in compounds, ionic and covalent. Ionic bonds result when electrons have been transferred between atoms, resulting in oppositely charged ions that attract each other. generally found when metal atoms bonded to nonmetal atoms ...
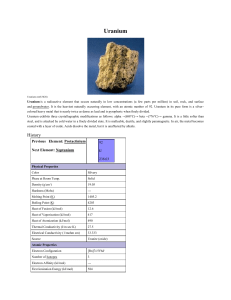
Uranium
... While thorium itself is not fissionable, 233U is, and in this way may be used as a nuclear fuel. One pound of completely fissioned uranium has the fuel value of over 1500 tons of coal. The uses of nuclear fuels to generate electrical power, to make isotopes for peaceful purposes, and to make explosi ...
... While thorium itself is not fissionable, 233U is, and in this way may be used as a nuclear fuel. One pound of completely fissioned uranium has the fuel value of over 1500 tons of coal. The uses of nuclear fuels to generate electrical power, to make isotopes for peaceful purposes, and to make explosi ...
Step 2
... structures built from a few hundred atoms and are 1100nm big. They show different properties to the same materials in bulk. They also have a large surface area to volume ratio and their properties could lead to new developments in computers, building materials etc. ...
... structures built from a few hundred atoms and are 1100nm big. They show different properties to the same materials in bulk. They also have a large surface area to volume ratio and their properties could lead to new developments in computers, building materials etc. ...
Step 2 - The Grange School Blogs
... structures built from a few hundred atoms and are 1100nm big. They show different properties to the same materials in bulk. They also have a large surface area to volume ratio and their properties could lead to new developments in computers, building materials etc. ...
... structures built from a few hundred atoms and are 1100nm big. They show different properties to the same materials in bulk. They also have a large surface area to volume ratio and their properties could lead to new developments in computers, building materials etc. ...
Chemical Equations and Stoichiometry
... The Mole: Glossary of Terms to Remember • Mass: depends on the amount of matter in a substance. Unlike weight it does not depend on gravity. In chemistry we most commonly measure mass in grams. • Mole: a package of 6.02 X 1023 items, usually molecules. Technically, it is the number of atoms found in ...
... The Mole: Glossary of Terms to Remember • Mass: depends on the amount of matter in a substance. Unlike weight it does not depend on gravity. In chemistry we most commonly measure mass in grams. • Mole: a package of 6.02 X 1023 items, usually molecules. Technically, it is the number of atoms found in ...
Group 1: The Alkali Metals
... The word "alkali" received its name from the Arabic word "al qali," meaning "from ashes". These particular elements were given the name "Alkali" because they react with water to form hydroxide ions, creating a basic solution (pH>7). Solutions that have a pH greater than 7 are called alkaline solutio ...
... The word "alkali" received its name from the Arabic word "al qali," meaning "from ashes". These particular elements were given the name "Alkali" because they react with water to form hydroxide ions, creating a basic solution (pH>7). Solutions that have a pH greater than 7 are called alkaline solutio ...
CHM 22 Test 2Take-homeKey Student Name
... 9. The following reaction: Mg + FeO MgO + Fe, is an example of A. combination. B. cecomposition. C. single-displacement. D. double-displacement. Answer: C; Difficulty: easy; Reference: Section 8.4 10. The following reaction: NaOH + HCl NaCl + H2O, is an example of A. combination. B. decomposition. C ...
... 9. The following reaction: Mg + FeO MgO + Fe, is an example of A. combination. B. cecomposition. C. single-displacement. D. double-displacement. Answer: C; Difficulty: easy; Reference: Section 8.4 10. The following reaction: NaOH + HCl NaCl + H2O, is an example of A. combination. B. decomposition. C ...
1 - Grygla School
... Using reactions to manufacture chemicals is a big industry. Table 1 lists the top eight chemicals made in the United States. Some of these chemicals may be familiar, and some you may have never heard of. By the end of this course, you will know a lot more about them. Chemicals produced on a small sc ...
... Using reactions to manufacture chemicals is a big industry. Table 1 lists the top eight chemicals made in the United States. Some of these chemicals may be familiar, and some you may have never heard of. By the end of this course, you will know a lot more about them. Chemicals produced on a small sc ...
O usually has oxidation number of -2, except in peroxides where it is
... O usually has oxidation number of -2, except in peroxides where it is assigned -1, and in OF2 where it is assigned a +2 due to the higher electro negativity of F. -In calcium oxide, CaO, oxide ion has a 2- charge. Its oxidation number is -2 -In H2O2, Each O is assigned the oxidation number of -1 5. ...
... O usually has oxidation number of -2, except in peroxides where it is assigned -1, and in OF2 where it is assigned a +2 due to the higher electro negativity of F. -In calcium oxide, CaO, oxide ion has a 2- charge. Its oxidation number is -2 -In H2O2, Each O is assigned the oxidation number of -1 5. ...
Practice Question
... chloride ions, and possibly bacteria, chlorine, and other ingredients. Which choice best defines what tap water is? ...
... chloride ions, and possibly bacteria, chlorine, and other ingredients. Which choice best defines what tap water is? ...
- Te Kura
... This topic consists of 10 lessons covering the fundamental concepts of curriculum level 7 chemistry. It is recommended that you complete this booklet to revise these concepts. If you feel confident that you have understood the concepts of a lesson, you can skip the activities. You are expected to co ...
... This topic consists of 10 lessons covering the fundamental concepts of curriculum level 7 chemistry. It is recommended that you complete this booklet to revise these concepts. If you feel confident that you have understood the concepts of a lesson, you can skip the activities. You are expected to co ...
THE MOLE - hrsbstaff.ednet.ns.ca
... a. 1.00 mol of ammonium chloride to formula units b. 2.5 mol of O3 to molecules c. 0.003 mol of cadmium to atoms 4. Make the following conversions: a. 200 x 1023 formula units of AgCl to moles b. 6.02 x 1025 atoms of nitrogen to moles c. 120.2 x 1015 molecules of H2 to moles 5. How many atoms are co ...
... a. 1.00 mol of ammonium chloride to formula units b. 2.5 mol of O3 to molecules c. 0.003 mol of cadmium to atoms 4. Make the following conversions: a. 200 x 1023 formula units of AgCl to moles b. 6.02 x 1025 atoms of nitrogen to moles c. 120.2 x 1015 molecules of H2 to moles 5. How many atoms are co ...
Mole Intro - hrsbstaff.ednet.ns.ca
... a. 1.00 mol of ammonium chloride to formula units b. 2.5 mol of O3 to molecules c. 0.003 mol of cadmium to atoms 4. Make the following conversions: a. 200 x 1023 formula units of AgCl to moles b. 6.02 x 1025 atoms of nitrogen to moles c. 120.2 x 1015 molecules of H2 to moles 5. How many atoms are co ...
... a. 1.00 mol of ammonium chloride to formula units b. 2.5 mol of O3 to molecules c. 0.003 mol of cadmium to atoms 4. Make the following conversions: a. 200 x 1023 formula units of AgCl to moles b. 6.02 x 1025 atoms of nitrogen to moles c. 120.2 x 1015 molecules of H2 to moles 5. How many atoms are co ...
PHYSICAL SETTING CHEMISTRY
... questions in this examination. Some questions may require the use of the Reference Tables for Physical Setting/Chemistry. You are to answer all questions in all parts of this examination according to the directions provided in the examination booklet. Your answer sheet for Part A and Part B–1 is the ...
... questions in this examination. Some questions may require the use of the Reference Tables for Physical Setting/Chemistry. You are to answer all questions in all parts of this examination according to the directions provided in the examination booklet. Your answer sheet for Part A and Part B–1 is the ...
ChemistryReview
... 72. If an atom has 34 protons and 40 neutrons, what is its mass number? 73. If an atom of tin has a mass number of 118 and an atomic number of 50, how many neutrons are in its nucleus? 74. In a periodic table that included electron dot diagrams, in which column would the diagrams contain more dots— ...
... 72. If an atom has 34 protons and 40 neutrons, what is its mass number? 73. If an atom of tin has a mass number of 118 and an atomic number of 50, how many neutrons are in its nucleus? 74. In a periodic table that included electron dot diagrams, in which column would the diagrams contain more dots— ...
Deans Community High School Intermediate 2 Revision Notes www
... The reason is that if we used an equal sign, we are saying that the products are the same as the reactants. This is not the case, as all chemical reactions produce a new substance. For example, the reaction between sodium metal and chlorine gas would be : sodium + chloride sodium chloride ...
... The reason is that if we used an equal sign, we are saying that the products are the same as the reactants. This is not the case, as all chemical reactions produce a new substance. For example, the reaction between sodium metal and chlorine gas would be : sodium + chloride sodium chloride ...
Mole Concept and Stoichiometry
... constructed that could determine if the sample was one atom over or under exactly 12 grams. If the first two requirements were met, it would take one million machines counting one million atoms each second more than 19,000 years to complete the task. So, practically it can be treated as impossible t ...
... constructed that could determine if the sample was one atom over or under exactly 12 grams. If the first two requirements were met, it would take one million machines counting one million atoms each second more than 19,000 years to complete the task. So, practically it can be treated as impossible t ...
Packet 4
... solvent, usually water). The concentration of a solution, can be measured in terms of the number of grams of the solute (solid) that has been dissolved in a particular volume of the solvent (usually water), or in terms of the number of moles of the solute in a particular volume of the solvent. Typic ...
... solvent, usually water). The concentration of a solution, can be measured in terms of the number of grams of the solute (solid) that has been dissolved in a particular volume of the solvent (usually water), or in terms of the number of moles of the solute in a particular volume of the solvent. Typic ...
The s-Block Elements
... Although lithium has highly negative Eo, it only reacts slowly with water. This illustrates the importance of the role of kinetic factors in determining the rate of a chemical reaction. Lithium has a higher m.p., this increases the activation energy required for dissolution in aqueous solution. It ...
... Although lithium has highly negative Eo, it only reacts slowly with water. This illustrates the importance of the role of kinetic factors in determining the rate of a chemical reaction. Lithium has a higher m.p., this increases the activation energy required for dissolution in aqueous solution. It ...
06.1 - Chemical formulas and composition stoichiometry
... Calcium oxide (CaO) is 71.5% calcium and 28.5% oxygen by mass Calcium carbonate (CaCO3) is 40.1% calcium, 12.0% carbon, 47.9% oxygen by mass ...
... Calcium oxide (CaO) is 71.5% calcium and 28.5% oxygen by mass Calcium carbonate (CaCO3) is 40.1% calcium, 12.0% carbon, 47.9% oxygen by mass ...
111 Review Outline TRO
... When most reactions are performed, some of the reactants is usually present in excess of the amount needed. If the reaction goes to completion, then some of this excess reactant will be left-over. The limiting reactant is the reactant used-up completely and it "limits" the reaction. For example: ...
... When most reactions are performed, some of the reactants is usually present in excess of the amount needed. If the reaction goes to completion, then some of this excess reactant will be left-over. The limiting reactant is the reactant used-up completely and it "limits" the reaction. For example: ...
Chapter 4
... that the masses of element Y that combine with a fixed mass of elements X to form two or more different compounds are in the ratios of small whole numbers. • Examples: NO, NO2, N2O, N2O5, etc. ...
... that the masses of element Y that combine with a fixed mass of elements X to form two or more different compounds are in the ratios of small whole numbers. • Examples: NO, NO2, N2O, N2O5, etc. ...
Section 2 Chemical Formulas and Equations
... chemical symbols and chemical formulas. Chemists use chemical equations to describe reactions. A chemical equation uses chemical symbols and formulas as a short way to describe a chemical reaction. Anyone around the world who understands chemical formulas can understand chemical equations. From Reac ...
... chemical symbols and chemical formulas. Chemists use chemical equations to describe reactions. A chemical equation uses chemical symbols and formulas as a short way to describe a chemical reaction. Anyone around the world who understands chemical formulas can understand chemical equations. From Reac ...
PHYSICAL CHEMISTRY ERT 108 Semester II 2010
... - Reactions where some of the species are gases (ex: combustion rxn) – studied in a constant-volume calorimeter - Reactions not involving gases – studied in a constantpressure calorimeter. ...
... - Reactions where some of the species are gases (ex: combustion rxn) – studied in a constant-volume calorimeter - Reactions not involving gases – studied in a constantpressure calorimeter. ...