some basic concepts of chemistry
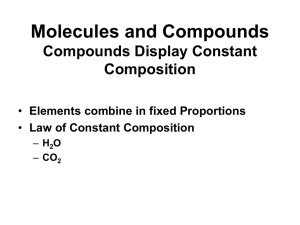
... different elements are present in a compound in a fixed and definite ratio and this ratio is characteristic of a particular compound. Also, the properties of a compound are different from those of its constituent elements. For example, hydrogen and oxygen are gases whereas the compound formed by the ...
... different elements are present in a compound in a fixed and definite ratio and this ratio is characteristic of a particular compound. Also, the properties of a compound are different from those of its constituent elements. For example, hydrogen and oxygen are gases whereas the compound formed by the ...
Chemistry MCQs - Target Publications
... With the change in educational curriculum it’s now time for a change in Competitive Examinations. NEET and ISEET are all poised to take over the decade old MHT-CET. The change is obvious not merely in the names but also at the competitive levels. The state level entrance examination is ushered aside ...
... With the change in educational curriculum it’s now time for a change in Competitive Examinations. NEET and ISEET are all poised to take over the decade old MHT-CET. The change is obvious not merely in the names but also at the competitive levels. The state level entrance examination is ushered aside ...
Redox Reactions - Hillsborough County Public Schools
... Examples: When calculating the oxidation number of N in NO2 , use the rules above to help you. You see that oxygen normally has an oxidation number of -2 and there are two oxygen atoms. 2(-2) = -4. The total number of negative charges is 4 negatives. The only other atom that is present is nitrogen. ...
... Examples: When calculating the oxidation number of N in NO2 , use the rules above to help you. You see that oxygen normally has an oxidation number of -2 and there are two oxygen atoms. 2(-2) = -4. The total number of negative charges is 4 negatives. The only other atom that is present is nitrogen. ...
Grade 11 Unit 8 - Amazon Web Services
... exist: organic substances, which were impossible to synthesize, and inorganic substances, which could be synthesized. Therefore, originally organic chemistry was founded as the study of compounds from living things that contained a vital spirit” (acquintessance). Historians of science refer to this ...
... exist: organic substances, which were impossible to synthesize, and inorganic substances, which could be synthesized. Therefore, originally organic chemistry was founded as the study of compounds from living things that contained a vital spirit” (acquintessance). Historians of science refer to this ...
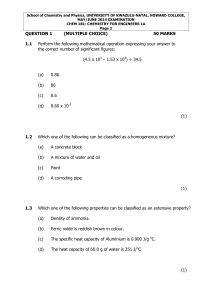
School of Chemistry
... School of Chemistry and Physics, UNIVERSITY OF KWAZULU-NATAL, HOWARD COLLEGE, MAY/JUNE 2014 EXAMINATION CHEM 181: CHEMISTRY FOR ENGINEERS 1A Page 10 ...
... School of Chemistry and Physics, UNIVERSITY OF KWAZULU-NATAL, HOWARD COLLEGE, MAY/JUNE 2014 EXAMINATION CHEM 181: CHEMISTRY FOR ENGINEERS 1A Page 10 ...
Chemistry English
... Why is Chemistry Interesting? Why is it necessary for you Chinese chemical students to learn Chemistry English? Completing both will be highly appreciated! ...
... Why is Chemistry Interesting? Why is it necessary for you Chinese chemical students to learn Chemistry English? Completing both will be highly appreciated! ...
2 - kcpe-kcse
... Calculating the formula from masses • When a new compound is discovered we have to deduce its formula. • This always involves getting data about the masses of elements that are combined together. • What we have to do is work back from this data to calculate the number of atoms of each element and t ...
... Calculating the formula from masses • When a new compound is discovered we have to deduce its formula. • This always involves getting data about the masses of elements that are combined together. • What we have to do is work back from this data to calculate the number of atoms of each element and t ...
Topic 2 notes - WordPress.com
... Note that state symbols are added to each substance: o s – solid o g – gas o l – pure liquid (e.g water) o aq – aqueous solution…formed when substances dissolve in water (e.g sodium hydroxide) Mass is conserved in chemical reactions: Atoms are NOT made or destroyed in a chemical reaction. They are o ...
... Note that state symbols are added to each substance: o s – solid o g – gas o l – pure liquid (e.g water) o aq – aqueous solution…formed when substances dissolve in water (e.g sodium hydroxide) Mass is conserved in chemical reactions: Atoms are NOT made or destroyed in a chemical reaction. They are o ...
June 01, 2008
... As a chemist working in the forensics department of the SAPS, you were called upon to identify an unknown powder that was found at 3 different crime scenes. Your preliminary examination of the powder samples obtained from Scene 1 and Scene 2 might suggest it being an illegal drug (a date-rape drug). ...
... As a chemist working in the forensics department of the SAPS, you were called upon to identify an unknown powder that was found at 3 different crime scenes. Your preliminary examination of the powder samples obtained from Scene 1 and Scene 2 might suggest it being an illegal drug (a date-rape drug). ...
C1 – Topic 2 notes - ARK Elvin Academy
... Note that state symbols are added to each substance: o s – solid o g – gas o l – pure liquid (e.g water) o aq – aqueous solution…formed when substances dissolve in water (e.g sodium hydroxide) Mass is conserved in chemical reactions: Atoms are NOT made or destroyed in a chemical reaction. They are o ...
... Note that state symbols are added to each substance: o s – solid o g – gas o l – pure liquid (e.g water) o aq – aqueous solution…formed when substances dissolve in water (e.g sodium hydroxide) Mass is conserved in chemical reactions: Atoms are NOT made or destroyed in a chemical reaction. They are o ...
Oxidation numbers
... electronegative element is assigned the negative oxidation state. Fluorine, the most electronegative element, is always assigned an oxidation state of "-1" when combined with any other element. Hydrogen, whenever it is combined in a molecule, is assigned an oxidation state of "+1". When hydrog ...
... electronegative element is assigned the negative oxidation state. Fluorine, the most electronegative element, is always assigned an oxidation state of "-1" when combined with any other element. Hydrogen, whenever it is combined in a molecule, is assigned an oxidation state of "+1". When hydrog ...
What Are Compounds? - Parma School District
... shared electrons are assumed to “belong” to the more electronegative atom in each bond. More-specific rules are provided by the following guidelines. 1. The atoms in a pure element have an oxidation number of zero. examples: all atoms in sodium, Na, oxygen, O2, phosphorus, P4, and sulfur, S8, have o ...
... shared electrons are assumed to “belong” to the more electronegative atom in each bond. More-specific rules are provided by the following guidelines. 1. The atoms in a pure element have an oxidation number of zero. examples: all atoms in sodium, Na, oxygen, O2, phosphorus, P4, and sulfur, S8, have o ...
Atomic Structure
... An unwanted side effect of this medicine is that it can cause the patient to have ‘wind’ (too much gas in the intestine). The equation below represents the reaction between calcium carbonate and hydrochloric acid (the acid present in the stomach). CaCO3 (s) + 2HCl (aq) →CaCl2 (aq) + H2O (l) + CO2 (g ...
... An unwanted side effect of this medicine is that it can cause the patient to have ‘wind’ (too much gas in the intestine). The equation below represents the reaction between calcium carbonate and hydrochloric acid (the acid present in the stomach). CaCO3 (s) + 2HCl (aq) →CaCl2 (aq) + H2O (l) + CO2 (g ...
Chapter 4 Nomenclature and Chemical Equations
... Procedure in Deducing Chemical Formulas for Ionic Compounds ...
... Procedure in Deducing Chemical Formulas for Ionic Compounds ...
Chemical Calculations, Chemical Equations
... Atoms forming negative ions always generate one, predictable kind (gaining all electrons to bring the s&p orbital sum to 8). However, some atoms can form more than one positively charged ion, having the ability to lose different amount of electrons each time. This behavior is difficult to predict, a ...
... Atoms forming negative ions always generate one, predictable kind (gaining all electrons to bring the s&p orbital sum to 8). However, some atoms can form more than one positively charged ion, having the ability to lose different amount of electrons each time. This behavior is difficult to predict, a ...
physical setting chemistry
... questions or answers prior to the examination and that you have neither given nor received assistance in answering any of the questions during the examination. Your answer sheet and answer booklet cannot be accepted if you fail to sign this declaration. Notice. . . A four-function or scientific calc ...
... questions or answers prior to the examination and that you have neither given nor received assistance in answering any of the questions during the examination. Your answer sheet and answer booklet cannot be accepted if you fail to sign this declaration. Notice. . . A four-function or scientific calc ...
Balancing Reaction Equations Oxidation State Reduction
... Balance each of these two reactions using the eight steps we discussed. Assume that the reactions take place in alkaline solution. ...
... Balance each of these two reactions using the eight steps we discussed. Assume that the reactions take place in alkaline solution. ...
Chemical Reactions and The Mole
... the HUMV vehicle and its tires. The HUMV weighs 6550 pounds. So, when Chevrolet calls Firestone and asks for tires they do not tell them the total weight of all the HUMVs they are making they tell the how many HUMVs they are making. Chevy would not call them and say we need tires for 32750000 lbs of ...
... the HUMV vehicle and its tires. The HUMV weighs 6550 pounds. So, when Chevrolet calls Firestone and asks for tires they do not tell them the total weight of all the HUMVs they are making they tell the how many HUMVs they are making. Chevy would not call them and say we need tires for 32750000 lbs of ...
Moles
... How many particles are in a mole (also known as 22.4 L of a gas at STP or the molar mass of a substance)? ...
... How many particles are in a mole (also known as 22.4 L of a gas at STP or the molar mass of a substance)? ...
2.5 THE NAMES AND FORMULAS OF COMPOUNDS
... explain many of the properties of ionic compounds, but they aren’t sufficient to explain the physical state of molecular compounds. If covalent bonds were the only forces at work, molecular compounds would all be gases, as there would be no attraction between the molecules strong enough to order the ...
... explain many of the properties of ionic compounds, but they aren’t sufficient to explain the physical state of molecular compounds. If covalent bonds were the only forces at work, molecular compounds would all be gases, as there would be no attraction between the molecules strong enough to order the ...
www.studyguide.pk
... insoluble radium sulfate which was converted into aqueous radium bromide. This solution was then electrolysed using a mercury cathode and a carbon anode. (a) Radium has chemical reactions that are typical of Group II metals and forms ionic ...
... insoluble radium sulfate which was converted into aqueous radium bromide. This solution was then electrolysed using a mercury cathode and a carbon anode. (a) Radium has chemical reactions that are typical of Group II metals and forms ionic ...
FoundationsofChemistryppt
... disagree with each of these statements. As you view this presentation, see if you change your mind about any of the statements. ...
... disagree with each of these statements. As you view this presentation, see if you change your mind about any of the statements. ...