
Solution of the 1st Major Exam, Term 061, Version 000, all correct
... Balance the following equation using the smallest set of whole numbers, then add together all the coefficients: SF4 + H2O H2SO3 + HF. The sum of the coefficients is A) 9 ...
... Balance the following equation using the smallest set of whole numbers, then add together all the coefficients: SF4 + H2O H2SO3 + HF. The sum of the coefficients is A) 9 ...
NCERT Solution - Mywayteaching
... combines with oxygen, the lattice energy of the oxide involving O2− ion is much more than the oxide involving O− ion. Hence, the oxide having O2− ions are more stable than oxides having O−. Hence, we can say that formation of O2− is energetically more favourable than formation of O−. ...
... combines with oxygen, the lattice energy of the oxide involving O2− ion is much more than the oxide involving O− ion. Hence, the oxide having O2− ions are more stable than oxides having O−. Hence, we can say that formation of O2− is energetically more favourable than formation of O−. ...
5073 Chemistry (SPA)
... For over 2000 years, people have wondered about the fundamental building blocks of matter. As far back as 440 BC, the Greek Leucippus and his pupil Democritus coined the term atomos to describe the smallest particle of matter. It translates to mean something that is indivisible. In the eighteenth ce ...
... For over 2000 years, people have wondered about the fundamental building blocks of matter. As far back as 440 BC, the Greek Leucippus and his pupil Democritus coined the term atomos to describe the smallest particle of matter. It translates to mean something that is indivisible. In the eighteenth ce ...
Atomic Mass - HCC Learning Web
... EX. 4 Consider the reaction, 2NH3 + 5F2 → N2F4 + 6HF. If 25.0 g of NH3 are reacted with 150. g of F2, (a) What is the limiting reactant? (b) Calculate the theoretical yield of N2F4 in grams. (c) Calculate the percent yield if 56.8 g of N2F4 are actually obtained. (d) Calculate the actual yield of N ...
... EX. 4 Consider the reaction, 2NH3 + 5F2 → N2F4 + 6HF. If 25.0 g of NH3 are reacted with 150. g of F2, (a) What is the limiting reactant? (b) Calculate the theoretical yield of N2F4 in grams. (c) Calculate the percent yield if 56.8 g of N2F4 are actually obtained. (d) Calculate the actual yield of N ...
Chem 11 Notes Booklet (pdf version)
... All matter is made up of about 100 elements. Elements are pure substances that cannot be broken down into simpler parts by ordinary chemical means. Each element is composed of a fundamental particle called an atom. Each element has a unique atom and is represented by a symbol (memorize the sheet “ ...
... All matter is made up of about 100 elements. Elements are pure substances that cannot be broken down into simpler parts by ordinary chemical means. Each element is composed of a fundamental particle called an atom. Each element has a unique atom and is represented by a symbol (memorize the sheet “ ...
Stage 2 Chemistry Intended Student Learning 2014
... Topic 1: Elemental and Environmental Chemistry This topic deals with some of the underlying principles of chemistry (‘elemental chemistry’) and then considers the chemistry of the environment. The elemental chemistry component of the topic focuses on the periodic table and the concept of electroneg ...
... Topic 1: Elemental and Environmental Chemistry This topic deals with some of the underlying principles of chemistry (‘elemental chemistry’) and then considers the chemistry of the environment. The elemental chemistry component of the topic focuses on the periodic table and the concept of electroneg ...
Note Sheets and Sample Problems
... 400 B.C.—Greeks—proposed all matter was make up of 4 “elements” : fire, earth, water and air Democritus—first to use the term atomos to describe the ultimate, smallest particles of matter Next 2,000 years—alchemy—a pseudoscience where people thought they could turn metals into gold. Some good chemis ...
... 400 B.C.—Greeks—proposed all matter was make up of 4 “elements” : fire, earth, water and air Democritus—first to use the term atomos to describe the ultimate, smallest particles of matter Next 2,000 years—alchemy—a pseudoscience where people thought they could turn metals into gold. Some good chemis ...
Chemical and physical changes
... A. ……………. changes are those in which the substances continue ............... the same ones. B. Chemical ……………….. are those in which the ……………….. that there are at the beginning ……………….. and in their place new ones appear. C. The ……………….. changes are called chemical ……………….. . D. Pure substances can ...
... A. ……………. changes are those in which the substances continue ............... the same ones. B. Chemical ……………….. are those in which the ……………….. that there are at the beginning ……………….. and in their place new ones appear. C. The ……………….. changes are called chemical ……………….. . D. Pure substances can ...
The Complete Notes - Joliet Junior College
... the course. Thus, it is important that the student does not let any ‘gaps’ in their knowledge develop. This fact exemplifies the differences in philosophy between the sciences and arts, as art courses are often more modular in nature. Example: I overhead a student tell another: “Yeah, I blew off rea ...
... the course. Thus, it is important that the student does not let any ‘gaps’ in their knowledge develop. This fact exemplifies the differences in philosophy between the sciences and arts, as art courses are often more modular in nature. Example: I overhead a student tell another: “Yeah, I blew off rea ...
5073 Chemistry IGCSE ordinary level for 2016
... For over 2000 years, people have wondered about the fundamental building blocks of matter. As far back as 440 BC, the Greek Leucippus and his pupil Democritus coined the term atomos to describe the smallest particle of matter. It translates to mean something that is indivisible. In the eighteenth ce ...
... For over 2000 years, people have wondered about the fundamental building blocks of matter. As far back as 440 BC, the Greek Leucippus and his pupil Democritus coined the term atomos to describe the smallest particle of matter. It translates to mean something that is indivisible. In the eighteenth ce ...
1001_3rd Exam_1001214
... Answer: A 29) The first ionization energy for rubidium is +403.0 kJ/mol. How much energy would be required to convert 17.1 g of gaseous rubidium to its gaseous +1 monatomic ion at constant temperature? A) 80.6 kJ B) 34.5 kJ C) 40.4 kJ D) 68.9 kJ E) 185 kJ Answer: A 30) Why is the electron affinity s ...
... Answer: A 29) The first ionization energy for rubidium is +403.0 kJ/mol. How much energy would be required to convert 17.1 g of gaseous rubidium to its gaseous +1 monatomic ion at constant temperature? A) 80.6 kJ B) 34.5 kJ C) 40.4 kJ D) 68.9 kJ E) 185 kJ Answer: A 30) Why is the electron affinity s ...
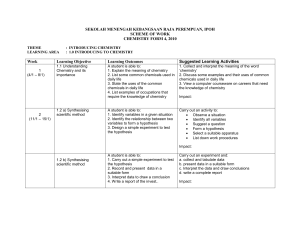
SEKOLAH MENENGAH KEBANGSAAN RAJA PEREMPUAN, IPOH
... properties of chlorine, bromine and iodine. 3. Describe changes in the physical properties from chlorine to iodine 4. List the chemical properties of chlorine, bromine and iodine 5. Describe the similarities in chemical properties of chlorine, bromine and iodine 6. Relate the chemical properties of ...
... properties of chlorine, bromine and iodine. 3. Describe changes in the physical properties from chlorine to iodine 4. List the chemical properties of chlorine, bromine and iodine 5. Describe the similarities in chemical properties of chlorine, bromine and iodine 6. Relate the chemical properties of ...
Notes-C12-121
... Organic and Inorganic Compounds Organic vs. Inorganic • Organic chemistry: Study of hydrocarbons (only carbon and hydrogen atoms) and their various derivatives. – Examples: natural gas, petroleum, plastics, rubbers, paper, carbohydrates (sugar, starch), proteins, enzymes, fatty acids, food stuff, dr ...
... Organic and Inorganic Compounds Organic vs. Inorganic • Organic chemistry: Study of hydrocarbons (only carbon and hydrogen atoms) and their various derivatives. – Examples: natural gas, petroleum, plastics, rubbers, paper, carbohydrates (sugar, starch), proteins, enzymes, fatty acids, food stuff, dr ...
Congratulations! You have signed up for AP Chemistry for this year
... 400 B.C.—Greeks—proposed all matter was make up of 4 “elements” : fire, earth, water and air Democritus—first to use the term atomos to describe the ultimate, smallest particles of matter Next 2,000 years—alchemy—a pseudoscience where people thought they could turn metals into gold. Some good chemis ...
... 400 B.C.—Greeks—proposed all matter was make up of 4 “elements” : fire, earth, water and air Democritus—first to use the term atomos to describe the ultimate, smallest particles of matter Next 2,000 years—alchemy—a pseudoscience where people thought they could turn metals into gold. Some good chemis ...
Chemistry 101: The Complete Notes
... the course. Thus, it is important that the student does not let any „gaps‟ in their knowledge develop. This fact exemplifies the differences in philosophy between the sciences and arts, as art courses are often more modular in nature. Example: I overhead a student tell another: “Yeah, I blew off rea ...
... the course. Thus, it is important that the student does not let any „gaps‟ in their knowledge develop. This fact exemplifies the differences in philosophy between the sciences and arts, as art courses are often more modular in nature. Example: I overhead a student tell another: “Yeah, I blew off rea ...
111 Exam I Outline
... a) Calculate the empirical formula of the compound. 6a) Ans: C2H8N3O b) What is the molecular formula of the compound? 6b) Ans: C4H16N6O2 7) 169 g FeCr2O4, 298 g K2CO3 and an excess of O2 (g) are sealed in a reaction vessel and allowed to react at high temperature. The amount of K2CrO4 obtained is 1 ...
... a) Calculate the empirical formula of the compound. 6a) Ans: C2H8N3O b) What is the molecular formula of the compound? 6b) Ans: C4H16N6O2 7) 169 g FeCr2O4, 298 g K2CO3 and an excess of O2 (g) are sealed in a reaction vessel and allowed to react at high temperature. The amount of K2CrO4 obtained is 1 ...
Regents Review Questions
... A substance known as heavy water can be obtained from ordinary water and could be a significant source of energy in the future. Heavy water contains deuterium, H-2. Instead of the two hydrogen atoms in a typical water molecule, a heavy water molecule has two deuterium atoms. In 3.78 kilograms of ord ...
... A substance known as heavy water can be obtained from ordinary water and could be a significant source of energy in the future. Heavy water contains deuterium, H-2. Instead of the two hydrogen atoms in a typical water molecule, a heavy water molecule has two deuterium atoms. In 3.78 kilograms of ord ...
111 Exam I Outline
... a) Calculate the empirical formula of the compound. 6a) Ans: C2H8N3O b) What is the molecular formula of the compound? 6b) Ans: C4H16N6O2 7) 169 g FeCr2O4, 298 g K2CO3 and an excess of O2 (g) are sealed in a reaction vessel and allowed to react at high temperature. The amount of K2CrO4 obtained is 1 ...
... a) Calculate the empirical formula of the compound. 6a) Ans: C2H8N3O b) What is the molecular formula of the compound? 6b) Ans: C4H16N6O2 7) 169 g FeCr2O4, 298 g K2CO3 and an excess of O2 (g) are sealed in a reaction vessel and allowed to react at high temperature. The amount of K2CrO4 obtained is 1 ...
Untitled - Frankedu
... (a) A substance is analyzed in a laboratory, and when viewed under an electron microscope, it is revealed that it contains only one kind of atom. Is the substance an element, compound or mixture? Ans: Element (b) Why do you think a chemical equation should be balanced? Ans: The Law of Conservati ...
... (a) A substance is analyzed in a laboratory, and when viewed under an electron microscope, it is revealed that it contains only one kind of atom. Is the substance an element, compound or mixture? Ans: Element (b) Why do you think a chemical equation should be balanced? Ans: The Law of Conservati ...
Reactions and Balancing
... form (Fe), and substance “B” is an element in molecular form (O2). The result is a direct chemical combination of the two elements (Fe2O3, iron (III) oxide, which is “rust”). * we knew to use iron (III) b/c 3 is the most common ion when we look at the oxidation state periodic table ...
... form (Fe), and substance “B” is an element in molecular form (O2). The result is a direct chemical combination of the two elements (Fe2O3, iron (III) oxide, which is “rust”). * we knew to use iron (III) b/c 3 is the most common ion when we look at the oxidation state periodic table ...
General and Organic Chemistry Review Primer
... The mass number of an element, measured in atomic mass units, is equal to the number of protons and neutrons. Calculating an element’s mass number is complicated by the existence of isotopes, atoms of an element with the same number of protons but different numbers of neutrons. Many naturally occurr ...
... The mass number of an element, measured in atomic mass units, is equal to the number of protons and neutrons. Calculating an element’s mass number is complicated by the existence of isotopes, atoms of an element with the same number of protons but different numbers of neutrons. Many naturally occurr ...
Elemental Analysis
... stoichiometry. After collection and drying, the product is weighed on an analytical balance and from the mass and known stoichiometry, the original analyte is quantitatively determined. Sometimes, an analyte is isolated in a form of a volatile compound. Gravimetry is an absolute (standard-free) meth ...
... stoichiometry. After collection and drying, the product is weighed on an analytical balance and from the mass and known stoichiometry, the original analyte is quantitatively determined. Sometimes, an analyte is isolated in a form of a volatile compound. Gravimetry is an absolute (standard-free) meth ...
Matter is anything that has mass and occupies space. Three
... Law of Definite Proportions: A given compound always contains exactly the same proportion of elements by weight. Law of Multiple Proportions: If two elements can combine to form more than one compound, then the masses of one element that combines with a fixed mass of the other element are in the rat ...
... Law of Definite Proportions: A given compound always contains exactly the same proportion of elements by weight. Law of Multiple Proportions: If two elements can combine to form more than one compound, then the masses of one element that combines with a fixed mass of the other element are in the rat ...
[SESSION-2014-2015] SUBJECT - SCIENCE PATNA REGION
... A water insoluble substance ‗X‘ on reacting with dilute H2SO4 released a colourless and odourless gas accompanied by brisk effervescence. When the gas was passed through water, the solution obtained turned blue litmus red. On bubbling the gas through lime water, it initially became milky and milkyne ...
... A water insoluble substance ‗X‘ on reacting with dilute H2SO4 released a colourless and odourless gas accompanied by brisk effervescence. When the gas was passed through water, the solution obtained turned blue litmus red. On bubbling the gas through lime water, it initially became milky and milkyne ...






















![[SESSION-2014-2015] SUBJECT - SCIENCE PATNA REGION](http://s1.studyres.com/store/data/008930072_1-5a35e1ae8e3204ea88999f1418a93013-300x300.png)