ch6 - ChemistryVCE
... b To what group of the periodic table do sodium and potassium both belong? c Suggest why potassium chloride is a suitable substitute for some of the sodium chloride. A15. a ...
... b To what group of the periodic table do sodium and potassium both belong? c Suggest why potassium chloride is a suitable substitute for some of the sodium chloride. A15. a ...
Beginning Chemistry
... combined in definite proportions by mass to give a material having a definite set of properties different from that of any of its constituent elements. For example, the compound water consists of 88.8 percent oxygen and 11.2 percent hydrogen by mass. The physical and chemical properties of water are ...
... combined in definite proportions by mass to give a material having a definite set of properties different from that of any of its constituent elements. For example, the compound water consists of 88.8 percent oxygen and 11.2 percent hydrogen by mass. The physical and chemical properties of water are ...
Atoms and Molecules
... electrons of an atoms can have are called energy levels or electron shells. • The first shell, closest to the nucleus, has the lowest potential energy. • Electrons in outer shells have more potential energy. • Electrons can only change their position if they absorb or release a quantity of energy th ...
... electrons of an atoms can have are called energy levels or electron shells. • The first shell, closest to the nucleus, has the lowest potential energy. • Electrons in outer shells have more potential energy. • Electrons can only change their position if they absorb or release a quantity of energy th ...
elements of chemistry unit
... One type of chemical reaction involves the transfer of electrons from one species (species means atoms or groups of atoms) to another. These reactions are called oxidation reduction reactions. The species that loses electrons is oxidized and the species gaining electrons is reduced. Oxidation reduct ...
... One type of chemical reaction involves the transfer of electrons from one species (species means atoms or groups of atoms) to another. These reactions are called oxidation reduction reactions. The species that loses electrons is oxidized and the species gaining electrons is reduced. Oxidation reduct ...
GROUP 13 ELEMENTS -THE BORON FAMILY -
... ºC), Al(660.4 ºC), Ga(29.78 ºC), In(152.6 ºC) and Tl(303 ºC), • The low melting point of Gallium is reflected in the unusual structure of the metal, which contains Ga2. Ga, In and Tl ( thallium develops a bluish tinge on oxidation.) are mechanically soft metals. Boron is a non-metallic gray powder. ...
... ºC), Al(660.4 ºC), Ga(29.78 ºC), In(152.6 ºC) and Tl(303 ºC), • The low melting point of Gallium is reflected in the unusual structure of the metal, which contains Ga2. Ga, In and Tl ( thallium develops a bluish tinge on oxidation.) are mechanically soft metals. Boron is a non-metallic gray powder. ...
1 Mole
... That’s right – open your book to page 323 and copy that table into your notes… go on… do it! This spot had better not be blank when I check your notebook! ...
... That’s right – open your book to page 323 and copy that table into your notes… go on… do it! This spot had better not be blank when I check your notebook! ...
Q1. This question is about the structure of atoms. (a) Choose words
... The atomic number of an atom is the number of ..................................... in its nucleus and is equal to the number of ..................................................... if the atom is not charged. ...
... The atomic number of an atom is the number of ..................................... in its nucleus and is equal to the number of ..................................................... if the atom is not charged. ...
Fundamentals
... charge (-1), their mass is small, ~1/2000 amu). If you want, you can imagine that the electrons “orbit” around the nucleus, like the planets around the sun. Example - Helium atom: 2 protons, 2 electrons, 2 neutrons, mass ~ 4 amu In an atom, the number of electrons is equal to the number of protons, ...
... charge (-1), their mass is small, ~1/2000 amu). If you want, you can imagine that the electrons “orbit” around the nucleus, like the planets around the sun. Example - Helium atom: 2 protons, 2 electrons, 2 neutrons, mass ~ 4 amu In an atom, the number of electrons is equal to the number of protons, ...
7 Periodic Properties of the Elements
... affinity, a gas phase single atom property, of F is less negative than that of Cl, the tendency of F to hold its own electrons (high ionization energy) coupled with a relatively large exothermic electron affinity makes it extremely susceptible to reduction and chemical bond formation. Cl is unreacti ...
... affinity, a gas phase single atom property, of F is less negative than that of Cl, the tendency of F to hold its own electrons (high ionization energy) coupled with a relatively large exothermic electron affinity makes it extremely susceptible to reduction and chemical bond formation. Cl is unreacti ...
Chapter 14
... We always need to know what the percentage of each element is in a given compound. This is very important to understand the exact composition of for example drugs, food, and many other things. The Percentage Composition of a compound can be calculated from the relative atomic masses of each element ...
... We always need to know what the percentage of each element is in a given compound. This is very important to understand the exact composition of for example drugs, food, and many other things. The Percentage Composition of a compound can be calculated from the relative atomic masses of each element ...
Chemistry - Kendriya Vidyalaya Raigarh
... a metallic element which forms the cations, (ii) High electron gain enthalpy of non- metallic element which forms the anions, (iii) Large lattice enthalpy i.e; the smaller size and the higher charge of the atoms. COVALENCY:The number of electrons which an atom contributes towards mutual sharing duri ...
... a metallic element which forms the cations, (ii) High electron gain enthalpy of non- metallic element which forms the anions, (iii) Large lattice enthalpy i.e; the smaller size and the higher charge of the atoms. COVALENCY:The number of electrons which an atom contributes towards mutual sharing duri ...
The science of chemistry is concerned with the
... As Lavoisier continued his experiments with oxygen, he noticed something else. Although oxygen combined with many other substances, it never behaved as though it were itself a combination of other substances. Lavoisier was able to decompose the red calx into mercury and oxygen, but he could find no ...
... As Lavoisier continued his experiments with oxygen, he noticed something else. Although oxygen combined with many other substances, it never behaved as though it were itself a combination of other substances. Lavoisier was able to decompose the red calx into mercury and oxygen, but he could find no ...
The science of chemistry is concerned with the composition
... As Lavoisier continued his experiments with oxygen, he noticed something else. Although oxygen combined with many other substances, it never behaved as though it were itself a combination of other substances. Lavoisier was able to decompose the red calx into mercury and oxygen, but he could find no ...
... As Lavoisier continued his experiments with oxygen, he noticed something else. Although oxygen combined with many other substances, it never behaved as though it were itself a combination of other substances. Lavoisier was able to decompose the red calx into mercury and oxygen, but he could find no ...
Chemical Quantities PPT
... atoms or formula units Since atoms are so small, extremely large numbers are needed in calculations Need to use a special counting unit just as used for other items A ream of paper One dozen donuts A pair of shoes ...
... atoms or formula units Since atoms are so small, extremely large numbers are needed in calculations Need to use a special counting unit just as used for other items A ream of paper One dozen donuts A pair of shoes ...
SrF 2(s)
... SR and DR reactions always happen in solutions so for ionic compounds check solubility table ...
... SR and DR reactions always happen in solutions so for ionic compounds check solubility table ...
AP Chem Stoichiometry Notes Table of Contents Atomic Masses
... Carvone is a substance that occurs in two forms having different arrangements of the atoms but the same molecular formula (C10H14O) and mass. One type of carvone gives caraway seeds their characteristic smell, and the other type is responsible for the smell of spearmint oil. Compute the mass percent ...
... Carvone is a substance that occurs in two forms having different arrangements of the atoms but the same molecular formula (C10H14O) and mass. One type of carvone gives caraway seeds their characteristic smell, and the other type is responsible for the smell of spearmint oil. Compute the mass percent ...
Chapter 5 notes
... The gives a formula of N2.173O4.347. Dividing both subscripts by the smaller of the two to get the smallest possible whole numbers (2.173/2.173 = 1, 4.347/2.173 ≈ 2) gives an empirical formula of NO2. Finally, dividing the approximate molar mass (92 g/mol) by the empirical formula ...
... The gives a formula of N2.173O4.347. Dividing both subscripts by the smaller of the two to get the smallest possible whole numbers (2.173/2.173 = 1, 4.347/2.173 ≈ 2) gives an empirical formula of NO2. Finally, dividing the approximate molar mass (92 g/mol) by the empirical formula ...
The Mole: A Measurement of Matter
... You live in a quantitative world. The grade you got on your last exam, the number of times you heard your favorite song on the radio yesterday, and the cost of a bicycle you would like to own are all important quantities to you. These are quantities that answer questions such as "How much?" or "How ...
... You live in a quantitative world. The grade you got on your last exam, the number of times you heard your favorite song on the radio yesterday, and the cost of a bicycle you would like to own are all important quantities to you. These are quantities that answer questions such as "How much?" or "How ...
physical setting chemistry
... the instructions from the proctor for completing the student information on your answer sheet. Record your answers to the Part A and Part B–1 multiple-choice questions on this separate answer sheet. Record your answers for the questions in Part B–2 and Part C in your separate answer booklet. Be sure ...
... the instructions from the proctor for completing the student information on your answer sheet. Record your answers to the Part A and Part B–1 multiple-choice questions on this separate answer sheet. Record your answers for the questions in Part B–2 and Part C in your separate answer booklet. Be sure ...
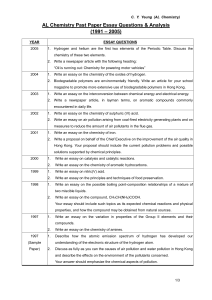
AL Chemistry Past paper essay questions
... Hong Kong. Your proposal should include the current pollution problems and possible solutions supported by chemical principles. ...
... Hong Kong. Your proposal should include the current pollution problems and possible solutions supported by chemical principles. ...
Chapter 3 Molecules, Compounds, and Chemical Equations
... Ionic Compounds • Compounds of metals with nonmetals are made of ions. Metal atoms: cations; nonmetal atoms: anions. • no individual molecule units, instead they have a three-dimensional array of cations and anions made of formula units • many contain polyatomic ions several atoms attached togethe ...
... Ionic Compounds • Compounds of metals with nonmetals are made of ions. Metal atoms: cations; nonmetal atoms: anions. • no individual molecule units, instead they have a three-dimensional array of cations and anions made of formula units • many contain polyatomic ions several atoms attached togethe ...
2nd Semester final review
... 1. What is a family or group on the periodic table and what is so important about it. Elements in a family or group (an up-down column) share similar chemical properties 2. Complete the chart below: Symbol Protons C-14 ...
... 1. What is a family or group on the periodic table and what is so important about it. Elements in a family or group (an up-down column) share similar chemical properties 2. Complete the chart below: Symbol Protons C-14 ...
Chemistry - Beachwood City Schools
... moving and far apart; aqueous (dissolved in water): particles moving and far. 5. chemical: Chlorine reacts with sodium to form NaCl. You could also list the formula of any ionic compound chlorine forms, such as MgCl2, CaCl2, etc. physical: Chlorine is a pale green gas at room temperature. It’s a non ...
... moving and far apart; aqueous (dissolved in water): particles moving and far. 5. chemical: Chlorine reacts with sodium to form NaCl. You could also list the formula of any ionic compound chlorine forms, such as MgCl2, CaCl2, etc. physical: Chlorine is a pale green gas at room temperature. It’s a non ...
Module 29: General Chemistry Instructor Guide – Answer Key
... area away from sources of heat, moisture and incompatibilities. Always add the caustic to water while stirring; never the reverse. Containers of this material may be hazardous when empty since they retain product residues (dust, solids); observe all warnings and precautions listed for the product. D ...
... area away from sources of heat, moisture and incompatibilities. Always add the caustic to water while stirring; never the reverse. Containers of this material may be hazardous when empty since they retain product residues (dust, solids); observe all warnings and precautions listed for the product. D ...
File
... Suppose that in one batch of reactants 4.20mol Al was mixed with 1.75mol Fe2O3. Which reactant, if any, was the limiting reactant? Calculate the mass of iron (in grams) that can be formed from this mixture of reactants. How do we approach this question – firstly determine what has been asked of you. ...
... Suppose that in one batch of reactants 4.20mol Al was mixed with 1.75mol Fe2O3. Which reactant, if any, was the limiting reactant? Calculate the mass of iron (in grams) that can be formed from this mixture of reactants. How do we approach this question – firstly determine what has been asked of you. ...