- TestbankU
... Chapter 2 The Chemical Context of Life 1) About twenty-five of the ninety-two natural elements are known to be essential to life. Which four of these twenty-five elements make up approximately 96 percent of living matter? A) carbon, sodium, hydrogen, nitrogen B) carbon, oxygen, phosphorus, hydrogen ...
... Chapter 2 The Chemical Context of Life 1) About twenty-five of the ninety-two natural elements are known to be essential to life. Which four of these twenty-five elements make up approximately 96 percent of living matter? A) carbon, sodium, hydrogen, nitrogen B) carbon, oxygen, phosphorus, hydrogen ...
CHM 151LL: States of Matter: Physical and Chemical Changes
... Substances can exist in three physical states: solid, liquid, and gas. Some of the differences between these states of matter are 1) the atoms’ or molecules’ freedom of movement and 2) the amount of space between the atoms or molecules. The physical state of a substance at a specific temperature dep ...
... Substances can exist in three physical states: solid, liquid, and gas. Some of the differences between these states of matter are 1) the atoms’ or molecules’ freedom of movement and 2) the amount of space between the atoms or molecules. The physical state of a substance at a specific temperature dep ...
Standard Enthalpy of Formation
... standard state at T is formed from the corresponding separated elements at T, each element being in its reference form. - The reference form (or reference phase) of an element at T is usually taken as the form of the element that is most stable at T and 1-bar pressure. ...
... standard state at T is formed from the corresponding separated elements at T, each element being in its reference form. - The reference form (or reference phase) of an element at T is usually taken as the form of the element that is most stable at T and 1-bar pressure. ...
Chapter 7: The Mole and Chemical Composition
... Notice that the unit of mole is abbreviated (mol), we like to abbreviate whenever possible in chemistry, even if it is only 1 letter… You can also use this same technique to convert from moles of an element or compound to grams of that element or compound. See…we use a balance to measure out grams ...
... Notice that the unit of mole is abbreviated (mol), we like to abbreviate whenever possible in chemistry, even if it is only 1 letter… You can also use this same technique to convert from moles of an element or compound to grams of that element or compound. See…we use a balance to measure out grams ...
Igcse chemistry lesson 2
... in this specification 1.19 use the state symbols (s), (l), (g) and (aq) in chemical equations to represent solids, liquids, gases and aqueous solutions respectively 1.20 understand how the formulae of simple compounds can be obtained experimentally, including metal oxides, water and salts containing ...
... in this specification 1.19 use the state symbols (s), (l), (g) and (aq) in chemical equations to represent solids, liquids, gases and aqueous solutions respectively 1.20 understand how the formulae of simple compounds can be obtained experimentally, including metal oxides, water and salts containing ...
Chemistry Entrance Material for Grade 11 to 12 Answer Key
... In all multiple choice questions, more than answer could be correct Section №: 1 Pure Substances Know where gaseous elements are located in the periodic table 01. What are the elements that are normally found as gases? H, N,O,F Cl, and Nobel gas 02. Where are these gaseous elements placed in the per ...
... In all multiple choice questions, more than answer could be correct Section №: 1 Pure Substances Know where gaseous elements are located in the periodic table 01. What are the elements that are normally found as gases? H, N,O,F Cl, and Nobel gas 02. Where are these gaseous elements placed in the per ...
Chapter One Chemistry
... characteristic mass more toand form substances— takes larger of aup pure particles space. substance called elements, molecules—groups compounds, that describes or its both—that ofability two or tomore are change together atoms into held in Chemistry is the study of the properties of matter and diffe ...
... characteristic mass more toand form substances— takes larger of aup pure particles space. substance called elements, molecules—groups compounds, that describes or its both—that ofability two or tomore are change together atoms into held in Chemistry is the study of the properties of matter and diffe ...
Chemical Equations and Reactions
... • If an atom appears more than once on a side, balance it last. • If you fix everything except one element, and it is even on one side and odd on the other, double the first number, then move on from there. C4H10 + O2 CO2 + H2O ...
... • If an atom appears more than once on a side, balance it last. • If you fix everything except one element, and it is even on one side and odd on the other, double the first number, then move on from there. C4H10 + O2 CO2 + H2O ...
Topic 1: Quantitative chemistry (12
... TOK: The early discoverers of the elements allowed chemistry to make great steps with limited apparatus, often derived from the pseudoscience of alchemy. Lavoisier’s work with oxygen, which overturned the phlogiston theory of heat, could be discussed as an example of a paradigm shift. Int: The disco ...
... TOK: The early discoverers of the elements allowed chemistry to make great steps with limited apparatus, often derived from the pseudoscience of alchemy. Lavoisier’s work with oxygen, which overturned the phlogiston theory of heat, could be discussed as an example of a paradigm shift. Int: The disco ...
FREE Sample Here
... Chapter 2 The Chemical Context of Life 1) About twenty-five of the ninety-two natural elements are known to be essential to life. Which four of these twenty-five elements make up approximately 96 percent of living matter? A) carbon, sodium, hydrogen, nitrogen B) carbon, oxygen, phosphorus, hydrogen ...
... Chapter 2 The Chemical Context of Life 1) About twenty-five of the ninety-two natural elements are known to be essential to life. Which four of these twenty-five elements make up approximately 96 percent of living matter? A) carbon, sodium, hydrogen, nitrogen B) carbon, oxygen, phosphorus, hydrogen ...
CHM 151LL: States of Matter: Physical and Chemical Changes
... Substances can exist in three physical states: solid, liquid, and gas. Some of the differences between these states of matter are 1) the atoms’ or molecules’ freedom of movement and 2) the amount of space between the atoms or molecules. The physical state of a substance at a specific temperature dep ...
... Substances can exist in three physical states: solid, liquid, and gas. Some of the differences between these states of matter are 1) the atoms’ or molecules’ freedom of movement and 2) the amount of space between the atoms or molecules. The physical state of a substance at a specific temperature dep ...
CHEMISTRY 101 Name Mock Final Exam Spring 2014 Signature Dr
... following statements must be true? a) Reactant A must be limiting. b) Reactant B must be limiting. c) If the coefficient for B is greater than the coefficient of A in the balanced ...
... following statements must be true? a) Reactant A must be limiting. b) Reactant B must be limiting. c) If the coefficient for B is greater than the coefficient of A in the balanced ...
CHAPTER 1 Differentiate b/w Mendeleev`s periodic law and modern
... Why atomic radii decrease from left to right in a period? Ans.The increase of nuclear charge and the no change of shielding effect decreases the atomic radii from left to right. Why atomic radii increase from top to bottom in a group? Ans.The increasing number of shells and increasing. shielding eff ...
... Why atomic radii decrease from left to right in a period? Ans.The increase of nuclear charge and the no change of shielding effect decreases the atomic radii from left to right. Why atomic radii increase from top to bottom in a group? Ans.The increasing number of shells and increasing. shielding eff ...
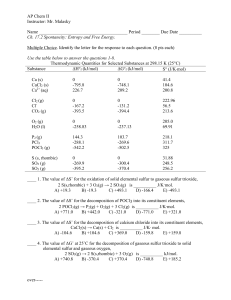
AP Chem II Instructor: Mr. Malasky Name Period ______ Due Date
... ____ 5. The value of ΔG˚ at 25˚C for the decomposition of gaseous sulfur dioxide to solid elemental sulfur and gaseous oxygen, SO2(g) → 2 S (s,rhombic) + O2(g) is __________ kJ/mol. A) +395.2 B) +269.9 C) -269.9 D) +300.4 E) -300.4 ____ 6. The value of ΔG˚ at 25˚C for the formation of POCl3 from it ...
... ____ 5. The value of ΔG˚ at 25˚C for the decomposition of gaseous sulfur dioxide to solid elemental sulfur and gaseous oxygen, SO2(g) → 2 S (s,rhombic) + O2(g) is __________ kJ/mol. A) +395.2 B) +269.9 C) -269.9 D) +300.4 E) -300.4 ____ 6. The value of ΔG˚ at 25˚C for the formation of POCl3 from it ...
chemistry - Textbooks Online
... Towards the end of the eighteenth century, pioneering work by Antoine and Marie Lavoisier and by John Dalton on the chemistry of air and the atomic nature of matter paved the way for modern chemistry. During the nineteenth century chemists worked steadily towards an understanding of the relationship ...
... Towards the end of the eighteenth century, pioneering work by Antoine and Marie Lavoisier and by John Dalton on the chemistry of air and the atomic nature of matter paved the way for modern chemistry. During the nineteenth century chemists worked steadily towards an understanding of the relationship ...
C - mvhs-fuhsd.org
... A. Atoms contain electrons. B. Practically all the mass of an atom is contained in its nucleus. C. Atoms contain protons, neutrons, and electrons. D. Atoms have a positively charged nucleus surrounded by an electron cloud. E. No two electrons in one atom can have the same four quantum numbers. 65. T ...
... A. Atoms contain electrons. B. Practically all the mass of an atom is contained in its nucleus. C. Atoms contain protons, neutrons, and electrons. D. Atoms have a positively charged nucleus surrounded by an electron cloud. E. No two electrons in one atom can have the same four quantum numbers. 65. T ...
formula
... Explain Millikan’s oil drop experiment & how it added to the atomic theory. Sketch the set-up used by Ernest Rutherford (the gold-foil experiment), show what he observed, and explain how these observations led to the idea that most of the mass of the atom is concentrated into a tiny, amazingly m ...
... Explain Millikan’s oil drop experiment & how it added to the atomic theory. Sketch the set-up used by Ernest Rutherford (the gold-foil experiment), show what he observed, and explain how these observations led to the idea that most of the mass of the atom is concentrated into a tiny, amazingly m ...
Glossary: Chemical bonds
... Cation. Compare with anion. A cation is a positively charged ion. Metals typically form cations. Chemical change. Reaction; chemical reaction. Compare with physical change. A chemical change is a dissociation, recombination, or rearrangement of atoms. compound Compare with element and mixture. A co ...
... Cation. Compare with anion. A cation is a positively charged ion. Metals typically form cations. Chemical change. Reaction; chemical reaction. Compare with physical change. A chemical change is a dissociation, recombination, or rearrangement of atoms. compound Compare with element and mixture. A co ...
A Review of High School Chemistry
... Copper (II) if it is +2 oxidation state Rule 3. The element with less metallic character is listed second. This second element is named by adding the suffix “ide” to the STEM of the element. Naming the nonmetals: ...
... Copper (II) if it is +2 oxidation state Rule 3. The element with less metallic character is listed second. This second element is named by adding the suffix “ide” to the STEM of the element. Naming the nonmetals: ...
Stoichometry Notes (Unit 2)
... The total number of atoms of each element (and the sum of their respective masses) on the reactant side of the “à” must be equal to the total number of atoms of each element (and the sum of their respective masses) on the product side. Chemical equations frequently contain additional symbols to repr ...
... The total number of atoms of each element (and the sum of their respective masses) on the reactant side of the “à” must be equal to the total number of atoms of each element (and the sum of their respective masses) on the product side. Chemical equations frequently contain additional symbols to repr ...
Electrochemistry
... A. Any chemical process in which electrons are transferred from one atom to another is an _________-__________ reaction. 1. The name for this type of reaction is often shortened to what is called a ________ reaction. 2. A species _____ _________ when _______ (LEO). A species _____ ________ when ____ ...
... A. Any chemical process in which electrons are transferred from one atom to another is an _________-__________ reaction. 1. The name for this type of reaction is often shortened to what is called a ________ reaction. 2. A species _____ _________ when _______ (LEO). A species _____ ________ when ____ ...
FREE Sample Here
... Chapter 2 The Chemical Context of Life 1) About twenty-five of the ninety-two natural elements are known to be essential to life. Which four of these twenty-five elements make up approximately 96 percent of living matter? A) carbon, sodium, hydrogen, nitrogen B) carbon, oxygen, phosphorus, hydrogen ...
... Chapter 2 The Chemical Context of Life 1) About twenty-five of the ninety-two natural elements are known to be essential to life. Which four of these twenty-five elements make up approximately 96 percent of living matter? A) carbon, sodium, hydrogen, nitrogen B) carbon, oxygen, phosphorus, hydrogen ...
Chemistry - RESONANCE PCCP IDEAL for NTSE, IJSO, Olympiads
... minimum value of atomic ratio as to get the simplest ratio of the atoms of elements present in the compound. (iii) If the simplest ratio is fractional, then values of simplest ratio of each element is multiplied by smallest integer to get the simplest whole number for each of the element. PAGE # 4 ...
... minimum value of atomic ratio as to get the simplest ratio of the atoms of elements present in the compound. (iii) If the simplest ratio is fractional, then values of simplest ratio of each element is multiplied by smallest integer to get the simplest whole number for each of the element. PAGE # 4 ...
State Standard - SchoolNotes.com
... Essential Question: How are atoms structured? How can atomic nuclei change? Understand: Chemical elements are the fundamental building materials of matter. Elemental properties are determined by the structure of the nucleus and distribution of electrons. One element can change into another through o ...
... Essential Question: How are atoms structured? How can atomic nuclei change? Understand: Chemical elements are the fundamental building materials of matter. Elemental properties are determined by the structure of the nucleus and distribution of electrons. One element can change into another through o ...