State Standard - SchoolNotes.com
... Essential Question: How are atoms structured? How can atomic nuclei change? Understand: Chemical elements are the fundamental building materials of matter. Elemental properties are determined by the structure of the nucleus and distribution of electrons. One element can change into another through o ...
... Essential Question: How are atoms structured? How can atomic nuclei change? Understand: Chemical elements are the fundamental building materials of matter. Elemental properties are determined by the structure of the nucleus and distribution of electrons. One element can change into another through o ...
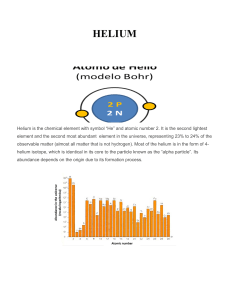
HELIUM - IDC
... There are eight known isotopes of helium, but only 3-Helium and 4-Helium Helium are stable. On Earth, 3Helium is present only in traces, mostly since the formation of the Earth. Helium has a valence of zero and is chemically non-reactive under normal conditions and is an electrical insulator unless ...
... There are eight known isotopes of helium, but only 3-Helium and 4-Helium Helium are stable. On Earth, 3Helium is present only in traces, mostly since the formation of the Earth. Helium has a valence of zero and is chemically non-reactive under normal conditions and is an electrical insulator unless ...
Matter and Measurement
... An atom is oxidized (loses electrons) if its oxidation number increases, and is reduced (gains electrons) if its oxidation number decreases An oxidizing agent causes the oxidation of another species by accepting an electron from it; in the process it is ...
... An atom is oxidized (loses electrons) if its oxidation number increases, and is reduced (gains electrons) if its oxidation number decreases An oxidizing agent causes the oxidation of another species by accepting an electron from it; in the process it is ...
Chapter 3: Stoichiometry
... • Compounds containing C, H and O are routinely analyzed through combustion in a chamber like this – C is determined from the mass of CO2 produced – H is determined from the mass of H2O produced – O is determined by difference after the C and H have been determined ...
... • Compounds containing C, H and O are routinely analyzed through combustion in a chamber like this – C is determined from the mass of CO2 produced – H is determined from the mass of H2O produced – O is determined by difference after the C and H have been determined ...
College Chemistry 1 Note Guide(free download)
... course for majors in the field. The DVDs are copies of those distance education videos and are made available courtesy of Gulf Coast Community College. The workbook/note guide written by Dr. Etheridge is designed to facilitate organized and complete note-taking to be used for study. The workbook mat ...
... course for majors in the field. The DVDs are copies of those distance education videos and are made available courtesy of Gulf Coast Community College. The workbook/note guide written by Dr. Etheridge is designed to facilitate organized and complete note-taking to be used for study. The workbook mat ...
PHYSICAL SETTING CHEMISTRY
... questions in this examination. Some questions may require the use of the Reference Tables for Physical Setting/Chemistry. You are to answer all questions in all parts of this examination according to the directions provided in the examination booklet. Your answer sheet for Part A and Part B–1 is the ...
... questions in this examination. Some questions may require the use of the Reference Tables for Physical Setting/Chemistry. You are to answer all questions in all parts of this examination according to the directions provided in the examination booklet. Your answer sheet for Part A and Part B–1 is the ...
A millennial overview of transition metal chemistry
... reasons. Their direct importance comes from their reactivity and the many chemical processes, including catalytic ones, in which they participate.11 Of course, it is not only metal carbonyls per se that are important but all of the many related low-valent complexes with ligands such as NO, phosphine ...
... reasons. Their direct importance comes from their reactivity and the many chemical processes, including catalytic ones, in which they participate.11 Of course, it is not only metal carbonyls per se that are important but all of the many related low-valent complexes with ligands such as NO, phosphine ...
Naming Binary Molecular Compounds
... An empirical formula consists of the symbols for the elements combined in a compound, with subscripts showing the smallest whole-number mole ratios of the different atoms in the compound. ...
... An empirical formula consists of the symbols for the elements combined in a compound, with subscripts showing the smallest whole-number mole ratios of the different atoms in the compound. ...
Campbell Biology, 10e (Reece) Chapter 2 The Chemical Context of
... Chapter 2 The Chemical Context of Life 1) About twenty-five of the ninety-two natural elements are known to be essential to life. Which four of these twenty-five elements make up approximately 96 percent of living matter? A) carbon, sodium, hydrogen, nitrogen B) carbon, oxygen, phosphorus, hydrogen ...
... Chapter 2 The Chemical Context of Life 1) About twenty-five of the ninety-two natural elements are known to be essential to life. Which four of these twenty-five elements make up approximately 96 percent of living matter? A) carbon, sodium, hydrogen, nitrogen B) carbon, oxygen, phosphorus, hydrogen ...
Chemistry 211 - George Mason University
... The Study of Chemistry • Chemistry = the study of the composition, properties and transformations of matter. • Matter = physical material of the universe. • Elements = basic building blocks of all other forms of matter. • Atoms = small particles derived from one the elements. All matter can be desc ...
... The Study of Chemistry • Chemistry = the study of the composition, properties and transformations of matter. • Matter = physical material of the universe. • Elements = basic building blocks of all other forms of matter. • Atoms = small particles derived from one the elements. All matter can be desc ...
OCR_AS_Level_Chemistry_Unit_F321_Atoms
... Oxidation states are also known as oxidation numbers Oxidation states are “charges” assigned to each element in a reaction The rules for assigning oxidation states are: o Elements are zero o In compounds, H is +1 and O is -2 o In compounds, Group 1 elements are +1, Group 2 are +2, Group 6 are -2 and ...
... Oxidation states are also known as oxidation numbers Oxidation states are “charges” assigned to each element in a reaction The rules for assigning oxidation states are: o Elements are zero o In compounds, H is +1 and O is -2 o In compounds, Group 1 elements are +1, Group 2 are +2, Group 6 are -2 and ...
Unit (1)
... 3- The nearest planet to the sun is ……………… and the farthest one from the sun is ……………… 4- Mercury, …………… , …………… and mars are the inner planets. 5- …………… planet has 27 moons revolving around it, while …………… planet has 12 moons revolving around it. 6- The comet consists of two parts which are …………… a ...
... 3- The nearest planet to the sun is ……………… and the farthest one from the sun is ……………… 4- Mercury, …………… , …………… and mars are the inner planets. 5- …………… planet has 27 moons revolving around it, while …………… planet has 12 moons revolving around it. 6- The comet consists of two parts which are …………… a ...
Diodes and Transistors HOW Theq Work
... silicon atoms in a crystal is four, because every atom forms four bonds. As mentioned previously, the electrons in the valence shell are called the atom's valence electrons. The rest of the atom, consisting of filled shells and the nucleus, is what is called the core. Remember, shells filled with el ...
... silicon atoms in a crystal is four, because every atom forms four bonds. As mentioned previously, the electrons in the valence shell are called the atom's valence electrons. The rest of the atom, consisting of filled shells and the nucleus, is what is called the core. Remember, shells filled with el ...
Module-2-s-and-d-elements - Львівський національний медичний
... view until the latter half of the 18th century. In 1781 the British chemist Henry Cavendish synthesized water by detonating a mixture of hydrogen and the air. However, the results of his experiments were not clearly interpreted until two years later, when the French chemist Antoine Laurent Lavoisie ...
... view until the latter half of the 18th century. In 1781 the British chemist Henry Cavendish synthesized water by detonating a mixture of hydrogen and the air. However, the results of his experiments were not clearly interpreted until two years later, when the French chemist Antoine Laurent Lavoisie ...
Chemistry Entrance Material for Grade 11 to 12
... Chemistry Entrance Material for Grade 11 to 12 ...
... Chemistry Entrance Material for Grade 11 to 12 ...
Unit 2: Practice
... 17. Determine the number of molecules found in 5.00 mol water. 18. Determine the number of carbon atoms found in 2.50 mol methane, CH4. 19. Calculate the number of moles of lead present in 8.6 1017 atoms of Pb. 20. Determine the molar mass of mercury(II) sulfide. 21. Ammonium carbonate is commonly ...
... 17. Determine the number of molecules found in 5.00 mol water. 18. Determine the number of carbon atoms found in 2.50 mol methane, CH4. 19. Calculate the number of moles of lead present in 8.6 1017 atoms of Pb. 20. Determine the molar mass of mercury(II) sulfide. 21. Ammonium carbonate is commonly ...
Chemistry - Gildredge House
... understanding of different areas of Chemistry and how they relate to each other. Students gain essential practical skills as well as a deep knowledge and understanding of scientific methods and competence in a variety of mathematical and problem solving skills. The course is designed and assessed ag ...
... understanding of different areas of Chemistry and how they relate to each other. Students gain essential practical skills as well as a deep knowledge and understanding of scientific methods and competence in a variety of mathematical and problem solving skills. The course is designed and assessed ag ...
Final Review 2006
... ____ 45. An element in the activity series can replace any element _____. a. in the periodic table. c. above it on the list. b. below it on the list. d. in its group. ____ 46. What can be predicted by using an activity series? a. whether a certain chemical reaction will occur b. the amount of energy ...
... ____ 45. An element in the activity series can replace any element _____. a. in the periodic table. c. above it on the list. b. below it on the list. d. in its group. ____ 46. What can be predicted by using an activity series? a. whether a certain chemical reaction will occur b. the amount of energy ...
Chemical Formulas and their arithmetic
... This stuff is important! It's not the most interesting part of chemistry, but it is by far the most fundamental in terms of most applications of the subject. Without a thorough understanding of the "chemical arithmetic" in this and the following lesson, you will find yourself stumbling through the r ...
... This stuff is important! It's not the most interesting part of chemistry, but it is by far the most fundamental in terms of most applications of the subject. Without a thorough understanding of the "chemical arithmetic" in this and the following lesson, you will find yourself stumbling through the r ...
Atomic structure and periodic table
... It can gain one extra electron to have stable electronic structure/configuration 2:8. Gaining requires less energy, and thus Fluorine reacts by gaining one extra electrons. 2. 2313 Al has electronic structure/configuration 2:8:3 It can donate the three outer electrons to have stable electronic struc ...
... It can gain one extra electron to have stable electronic structure/configuration 2:8. Gaining requires less energy, and thus Fluorine reacts by gaining one extra electrons. 2. 2313 Al has electronic structure/configuration 2:8:3 It can donate the three outer electrons to have stable electronic struc ...
File
... m) Non-metal: any element that is found on the right-hand side of the staircase line on the Periodic Table; tends to gain or share electrons to complete a stable octet electron arrangement. n) Metalloid: an element that is found close to the staircase line on the Periodic Table and has properties o ...
... m) Non-metal: any element that is found on the right-hand side of the staircase line on the Periodic Table; tends to gain or share electrons to complete a stable octet electron arrangement. n) Metalloid: an element that is found close to the staircase line on the Periodic Table and has properties o ...
File - Mc Guckin Science
... m) Non-metal: any element that is found on the right-hand side of the staircase line on the Periodic Table; tends to gain or share electrons to complete a stable octet electron arrangement. n) Metalloid: an element that is found close to the staircase line on the Periodic Table and has properties o ...
... m) Non-metal: any element that is found on the right-hand side of the staircase line on the Periodic Table; tends to gain or share electrons to complete a stable octet electron arrangement. n) Metalloid: an element that is found close to the staircase line on the Periodic Table and has properties o ...
ESO - ENCIGA
... tested by subsequent investigation and can be modified by its results. Science does not give statements of absolute eternal truth, it only provides theories. We know that those theories will probably be refined in the future, and some of them may even be discarded in favour of theories that make mor ...
... tested by subsequent investigation and can be modified by its results. Science does not give statements of absolute eternal truth, it only provides theories. We know that those theories will probably be refined in the future, and some of them may even be discarded in favour of theories that make mor ...
Chapter 3 - Whitwell High School
... • When you mix the tasty pancakes, do you always make the amount that the box predicts is possible? • Or, when baking cookies…it says you can make 3 dozen do you really? Or do you eat some dough? ...
... • When you mix the tasty pancakes, do you always make the amount that the box predicts is possible? • Or, when baking cookies…it says you can make 3 dozen do you really? Or do you eat some dough? ...
RES8_chemcontentchecklist
... Explain that a salt is produced when the H+ ion of an acid is replaced by a metal ion or NH4+. Describe the reactions of an acid with carbonates, bases and alkalis, to form a salt. Explain that a base readily accepts H+ ions from an acid: e.g. OH– forming H2O; ...
... Explain that a salt is produced when the H+ ion of an acid is replaced by a metal ion or NH4+. Describe the reactions of an acid with carbonates, bases and alkalis, to form a salt. Explain that a base readily accepts H+ ions from an acid: e.g. OH– forming H2O; ...