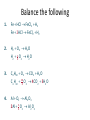* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download File
Electron configuration wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Strengthening mechanisms of materials wikipedia , lookup
Resonance (chemistry) wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Computational chemistry wikipedia , lookup
History of chemistry wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Biochemistry wikipedia , lookup
Implicit solvation wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Isotopic labeling wikipedia , lookup
Metalloprotein wikipedia , lookup
Chemical bond wikipedia , lookup
Bose–Einstein condensate wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Stoichiometry wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Solids, Liquids and Gases • Solids (s): • Liquids (l): • Gases (s): Molecules are held in place by intermolecular interactions. Molecules are held next to one another by intermolecular interactions, however, these interactions are not strong enough to prevent the molecules from flowing past one another. The intermolecular interactions are too weak to hold the molecules next to one another, so the molecules wander off on their own. Diffusion Diffusion is the spontaneous spreading out of a substance, and is due to the natural movement of its particles. Cotton wool soaked with hydrochloric acid Ammonium chloride Cotton wool soaked with ammonia solution Diffusion of ammonia and hydrogen chloride gases Ink being diffused in water The mass of atoms are very small • Therefore scientists cannot count individual atoms or molecules directly • Instead a scientist count there particles by weighing a certain number of them • Same as a bank cashier, by knowing the weight of one coin they can calculate the number of coins by dividing the total mass by the mass of one coin. A. What is the Mole? • A counting number (like a dozen) • Avogadro’s number (NA) • 1 mol = 6.02 1023 items A large amount!!!! The Mole • The mole is simply a chemist’s measurement for the amount of a substance. • It is a very large number (6 x 1023) as it ‘counts’ very small ‘objects’ such as atoms and molecules which have a very small masses. • This rather strange number was not selected deliberately – instead, it is the number of atoms in a sample of any element that has a mass in grams that is numerically equal to the elements atomic mass. • It allows chemists to work with masses in the lab which are feasible. The Mole Definition A mole of any substance is defined as the amount of the substance that contains as many particles as 12 g of carbon – 12. B. Molar Mass • Mass of 1 mole of an element or compound. • Atomic mass tells the... – – – – That 6.01 *1023 atoms will weigh this amount 6.01 *1023 atoms of carbons will weigh 12 grams grams per mole (g/mol) 1 mole of carbon is 12 grams per litre • Round to 2 decimal places B. Molar Mass Examples • carbon 12.01 g/mol • aluminum 26.98 g/mol • zinc 65.39 g/mol B. Molar Mass Examples • water – 2(1.01) + 16.00 = 18.02 g/mol – H2O • sodium chloride – NaCl – 22.99 + 35.45 = 58.44 g/mol B. Molar Mass Examples • sodium bicarbonate – NaHCO3 – 22.99 + 1.01 + 12.01 + 3(16.00) = 84.01 g/mol • sucrose – C12H22O11 – 12(12.01) + 22(1.01) + 11(16.00) = 342.34 g/mol If a magnesium atom is twice as heavy as an atom of carbon Then 100 moles of magnesium Are twice as heavy as 100 moles Of carbon Therefore if we have a piece of Magnesium that is twice as heavy As a piece of carbon they have an Equal number of atoms • Atom of carbon weighs • Mr=12 • Atom of hydrogen weighs • Mr=1 One gram of hydrogen atoms and twelve grams of Carbon atoms both have the same number of atoms • Atom of silver weighs • Ar=108 • Atom of carbon weighs • Ar=12 If we have 12g of carbon how do we calulate the mass of silver if it has the same number of atoms 12g 24g 27g 40g carbon magnesium aluminium calcium 56g 63.5 iron copper silver All these masses contain the same number of atoms Avogadro`s number = 6 X 1023 Symbol L It is the number of atoms of carbon in 12g of the carbon-12 isotope 108g The amount of a substance which contains 6 X 1023 particles is called a mole of that substance Symbol mol • One mole of carbon weighs 12grams • One mole of carbon contains 6 X 23 10 atoms • 48g of Mg (one mole weighs 24 g) • Is 2 moles of Mg Converting moles to grams • Mass of one mole of an element= relative atomic mass in grams Element H C N Mg Cu Fe I Relative atomic mass 1 12 14 24 63.5 56 127 The mass of one mole of an element = Relative Atomic Mass in grams The relative molecular mass of a compound is the sum of the relative atomic masses of all the atoms in the compound H2O = 2(1) + (16) = 18 (relative molecular mass of H2O) CuSO4.H2O =(63.5) + (32) +4(16) +5 (18)=249.5 Converting Grams to moles • 12g of carbon = 1 mole • 1g of carbon = 1/12 moles • 36g of carbon= 36/12=3 moles How many moles of sulfuric acid (H2SO4) are there in 12.25 g of sulfuric acid • Relative mol mass =2(1) + (32) + 4(16) = 98 • Total mass/mass of one mole • 12.25/98=0.125 mole Converting moles to number of atoms or molecules • How many atoms of sodium are present in 0.25 moles of a metal • 1 mole of Na contained 6x1023 atoms • 0.25 x 6x1023 atoms= 1.5 x1023 atoms (i) How many molecules are there in 0.5 mols of chlorine gas? (ii) How many atoms are there in 2 mols of water? (iii) How many electrons are there in 1.5 mols of Calcium? (i) 1 mol of Cl2 6 1023 molecules [Cl2 is composed of molecules] 0.5 mols of Cl2 0.5 6 1023 3 1023 molecules (ii) 1 mol of H2 0 6 1023 molecules [H2 O is composed of molecules] 2mols of H2 0 2 6 1023 1.2 1024 molecules Each molecule of water contains 3 atoms 1.2 1024 molecules contains 3 1.2 1024 3.6 1024 atoms. (ii) 1 mol of Ca 6 1023 atoms [Ca is composed of atoms] 1.5 mols of H2 0 1.5 6 1023 9 1023 atoms Each atom of Calcium contains 20 electrons 9 1023 atoms contain 23 9 1024 2.07 1025 electrons. Molar Volume • One mole of any gas should occupy the same volume as one mole of any other gas at the same conditions of temperature and pressure. • Gases are often compared at STP, standard temperature and pressure. Standard pressure = 101325 Pa Standard temperature = 273 K • Molar Volume = 22.4 L = 22,400 cm3 = 2.24 x 10–2 m3 (i) What is the volume in litres at STP of 0.01 mols of methane (CH4 ) gas? (ii) How many mols are there in 280 cm3 of fluorine gas at STP? (i) 1 mol of CH4 22400 cm3 0.01mols of CH4 0.01 22400 224 cm3 (ii) 1 mol of F2 22400 cm3 1 mols of F2 = 1cm3 22400 1 280 mols of F2 = 280cm3 22400 0.0125 molsof F2 280cm3 Relative Molecular Mass The relative molecular mass is the average mass of a molecule relative to one twelfth the mass of the carbon 12 atom. The relative molecular mass has no units as it is the ratio of two masses. (i) Calculate the relative molecular mass of (a) sulfuric acid, (H2 SO4 ) (b) Oxygen, (O2 ). (i) (a) H2 SO4 2(1) 32 4(16) 98 (b) O2 2(16) 32 Molar Mass • The molar mass of a substance is the mass in grams of one mole of the substance. • The molar mass has the same numerical value as its relative molecular mass, but its units are grams (g). Substance Relative molecular mass Molar mass Sulfuric aicd, H2 SO4 98 98 g Glucose, C6H12 O6 180 180 g Oxygen, O2 32 32 g Sulfur dioxide, SO2 64 64 g 1 mol of carbon, 12 g (i) How many moles are there in 990 g of cabon dioxide (CO2 ) (ii) The daily intake of calcium for an adult is 800 mg, how many moles of calcium is this? (i) 1 mol of CO2 44 g 1 mols of CO2 1g 44 1 990 mols of CO2 990 g 44 22.5 mols of CO2 990 g (ii) 1 mol of Ca 40 g 1 mols of Ca 1g 40 1 800 10 3 mols of Ca 800 10 3 g 40 0.02 mols of Ca 800 10 3 g [800mg 800 10 3 g] More Mole Calculations Volume of X Number of particles of X (if a gas at STP) in litres at STP Moles of X x molar mass ÷ molar mass Mass of X in g Notice that there is no direct link from particles to grams, you must first convert to moles. Likewise going from volume to mass. What is the mass of one atom of calcium? 1 mol of Ca 40 g 1 mol of Ca 6 1023 atoms 6 10 atoms 40 g 23 40 23 1 atom 6.67 10 g 23 6 10 The Nissan Micra 1.5 Diesel SVE is quoted to have a CO2 emission figure of 120 g / km. For a 40 km round trip to work calculate: (i) the mass of CO2 produced. (ii) the number of moles of CO2 produced. (iii) the volume of CO2 produced at room temperature and pressure. [Molar volume at room temperature and pressure 24.0 litres] (i) 120 40 4800 km (ii) no. of mols (iii) 1mol of CO2 24.0 L actual mass molecular mass 4800 no. of mols mols 44 no. of mols 109.09 mols 109.09 mols of CO2 109.09 24L 109.09 mols of CO2 2594.16 L How many iron atoms should be consumed daily to meet the recommended daily intake of iron in the diet of 0.014 g? [H2007, Q4 (e)] no. of mols acutalmass molecular mass 0.014 56 no. of mols 2.5 10 4 mols no. of mols 1mol 6 10 3 atoms 2.5 10 4 mols 2.5 10 4 6 10 3 atoms 2.5 10 4 mols 1.5 10 0 atoms Chemical Formulas Empirical Formula • The empirical formula of a compound is the formula that gives that simplest whole number ratio in which the atoms of the elements in the compound are present. • The empirical formula of glucose, C6H12O6 (ratio of atoms is 6:12:6) is CH2O, as this is the lowest ratio (1:2:1) of the atoms C, H and O. A sample of a brown coloured gas that is a major pollutant is found to contain 2.34 g of N and 5.34 g of O. What is the simplest formula of the compound? 2.34 mols of N atoms 0.167 14 5.34 mols of O atoms 0.334 16 Ratio of Nitrogen atoms to Oxygen atoms 1: 2 NO2 A dry - cleaning fluid composed of carbon and chlorine was found to have the composition 14.5% C, 85.5% Cl. What is the empirical formula of this compound? Element Percentage Percentage Simplest Ar Ratio C 14.5% Cl 85.5% CCl2 14.5 1.208 12 85.5 2.408 35.5 1 2 A 1.28 g sample of sulfur was allowed to react with an excess of chlorine to produce 4.12 g of a product that contains only sulfur and chlorine. What is the empirical formula of the compound? Mass of sulfur consumed = 1.28 g Mass of chlorine consumed = 4.12 1.28 2.84 g Mols of sulfur consumed = Mols of chlorine comsumed = Ratio of sulfur atoms to chlorine atoms = SCl2 1.28 0.04 32 2.84 0.08 35.5 1:2 Molecular Formula The molecular formula of a compound is the formula that gives the actual number of atoms of each element present in a molecule of the compound. A colourless liquid used in rocket engines, whose empirical formula is NO2 ,has molecular mass of 92. What is the molecular formula? Formula mass 46 Molecular mass 92 92 2 46 Molecular formula NO2 2 N2 O4 No. of NO2 in formula = Percentage Composition by Mass If the empirical formula of a compound is known, the percentage by mass of each element present can be calculated. It can be useful to calculate the percentage of a certain element in a compound, for example the percentage of nitrogen in fertilisers. Calculate the percentage by mass of nitrogen present in urea, CO(NH2 )2 . % of N mass of nitrogen 100 Mr of Urea 2(14) % of N 100 12 16 2[14 2(1)] 28 % of N 100 46.66% 60 Structural Formulas The structural formula of a compound shows the arrangement of the atoms within a molecule of the compound. Some Examples: H H H C C H H Methane, CH4 H H H C H Ethene, C2H4 H H C C H H OH Ethanol, C2H5OH You will come across many more structural formulas in the organic section of the course. Chemical Equations Chemical Equations • A chemical equation is a way of representing a chemical change. • It shows reactants and products. • To balance an equation means to change the numbers of each molecule involved, so that the same number of atoms of each element appear on the reactants side and on the products side. • Chemical equations balance on an atomic level, not molecular. • You cannot change the formula of a substance, i.e. if the equation has NH3 you cannot change this you can only put a number in front of it, 2NH3, increasing the number of N’s and the number of H’s. • Never change the subscripts (small numbers). • It is possible to write balanced equations for lots of reactions but that does not mean that the reaction actually takes place. Formation of Water (Oxygen does not exist as single atoms) (This reaction does not take place) Balancing an Equation Methane reacts with oxygen, forming carbon dioxide and water vapour only. Write a balanced chemical equation for this reaction. Solution CH4 O2 CO2 H2 O Examine each element in turn, to have the same no. of atoms of each on either side. (Leave the O until last as it appears the most often) Examining C: 1 on LHS and 1 on RHS, leave as they are. Examining H: 4 on LHS and 2 on RHS, put a 2 in front of the H2 O, on the RHS. CH4 O2 CO2 2H2 O Examining O: 2 on LHS and 4 on RHS, put a 2 in front of the O2 , on the LHS. CH4 2O2 CO2 2H2 O Balance the following 1. Fe HCl FeCl2 H2 Fe 2HCl FeCl2 H2 2. H2 O2 H2 O H2 12 O2 H2 O 3. C 4 H10 O2 CO2 H2 O C 4 H10 132 O2 4CO2 5H2 O 4. Al O2 Al2 O3 2Al 32 O2 Al2 O3 Balancing Redox Equations • In working out what is oxidised and what is reduced in a reaction, it is important to remember that oxidation numbers are not a charge. • Write the oxidation numbers below each atom to which it applies, as shown in the examples. H2 O 1 2 Cr2 O7 2 6 2 OH 2 1 • Oxidation is an increase in oxidation number. • Reduction is a decrease in oxidation number. Balance the following redox equation Cr2 O72 Fe2 H Cr 3 Fe3 H2O 1. Cr2 O7 2 Fe2 H Cr 3 Fe3 H2 O 2. Cr 3e Cr 3 Cr2 6e 2Cr 3 Fe2 e Fe3 Fe2 e Fe3 6 2 2 6 3 2 3. Cr2 6Fe2 1 3 6e 6e Cr2 6Fe2 3 3 1 2 3 6 2 Write down half equations of what is oxidised and reduced. Attach subscripts to atoms oxidised and reduced and balance the half equations. 3 2Cr 3 6Fe3 Assign oxidation numbers to all the atoms in the equation. Balance the half equations. 2Cr 3 6Fe3 4. Cr2 O72 6Fe2 H 2Cr 3 6Fe3 H2 O Attach species that were attached to the oxidised and reduced atoms. 5. Cr2 O72 6Fe2 14H 2Cr 3 6Fe3 7H2 O Include all the original species and complete the balancing by inspection. Calculations using Chemical Equations • A balanced equation for a chemical reaction gives the relative amounts of each reactant and each product involved in the reaction. • If the amount of one substance is known, based on the molar ratios in the equation, the amounts (masses, particles or volume)of other substances can be calculated. • Make sure to always work in moles. A dry - cleaning fluid composed of carbon and chlorine was found to have the composition 14.5% C, 85.5% Cl. What is the empirical formula of this compound? Element Percentage Percentage Simplest Ar Ratio C 14.5% Cl 85.5% CCl2 14.5 1.208 12 85.5 2.408 35.5 1 2