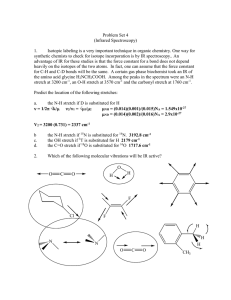
Chemdraw B&W - Pennsylvania State University
... • Alkylation occurs when the nucleophilic enolate ion reacts with the electrophilic alkyl halide or tosylate and displaces the leaving group ...
... • Alkylation occurs when the nucleophilic enolate ion reacts with the electrophilic alkyl halide or tosylate and displaces the leaving group ...
Lecture 03B - Balancing Redox
... -Halogens usually -1, unless combine with more EN element (dichlorine monoxide, ClO-Cl, Cl is +1; F is always -1) Rule 4: The algebraic sum of all O.N.s of all atoms in a neutral compound or polyatomic ion is equal to the net charge. - For neutral compounds, the sum of the O.N.s is 0 - For a charged ...
... -Halogens usually -1, unless combine with more EN element (dichlorine monoxide, ClO-Cl, Cl is +1; F is always -1) Rule 4: The algebraic sum of all O.N.s of all atoms in a neutral compound or polyatomic ion is equal to the net charge. - For neutral compounds, the sum of the O.N.s is 0 - For a charged ...
4.5: Bonding in Alcohols and Alkyl Halides
... 3°: three alkyl groups 2°: two alkyl groups donating electrons donating electrons ...
... 3°: three alkyl groups 2°: two alkyl groups donating electrons donating electrons ...
Heterocyclic Chemistry
... Nomenclature of heterocyclic compounds There are three systems for naming heterocylic compounds: 1) The common nomenclature: which convey little or no structural information but it still widely used. 2) The Hantzsch-Widman (IUPAC or Systematic) method which in contrast is designed so that one may ...
... Nomenclature of heterocyclic compounds There are three systems for naming heterocylic compounds: 1) The common nomenclature: which convey little or no structural information but it still widely used. 2) The Hantzsch-Widman (IUPAC or Systematic) method which in contrast is designed so that one may ...
Grant MacEwan College - Faculty Web Pages
... Description: This is the second course in organic chemistry. The topics covered include structural and chemical properties of alkenes, alkynes, alcohols, phenols, ethers, aromatic compounds. Aldehyde, ketones, amines, carboxylic acids, and carboxylic acid derivatives. Illustration of these functiona ...
... Description: This is the second course in organic chemistry. The topics covered include structural and chemical properties of alkenes, alkynes, alcohols, phenols, ethers, aromatic compounds. Aldehyde, ketones, amines, carboxylic acids, and carboxylic acid derivatives. Illustration of these functiona ...
Chap 1 - Notes - StrucandPropOrganicComp
... Recall: Carbon has four valence electrons (Electron Configuration: 1s22s22p2). This means that the outer valence shell is exactly half full and therefore carbon can form 4 covalent bonds. These outer electrons can hybridize to sp3, sp2, and sp orbitals, which means they can form single, double and ...
... Recall: Carbon has four valence electrons (Electron Configuration: 1s22s22p2). This means that the outer valence shell is exactly half full and therefore carbon can form 4 covalent bonds. These outer electrons can hybridize to sp3, sp2, and sp orbitals, which means they can form single, double and ...
Abstract OXIDATIVE TRANSFORMATIONS AND CYCLIZATIONS
... 20 mol% led to an improvement in the product formation. In all the cases, nitro derivatives are found to be the sole products. This methodology has been applied to heterocyclic compounds taking 3-aminoquinoline as an example. Section II. Mild and Efficient Benzylic Oxidation Catalyzed by KI and aque ...
... 20 mol% led to an improvement in the product formation. In all the cases, nitro derivatives are found to be the sole products. This methodology has been applied to heterocyclic compounds taking 3-aminoquinoline as an example. Section II. Mild and Efficient Benzylic Oxidation Catalyzed by KI and aque ...
oxidation numbers
... 1 Work out the formula of the species before and after the change; 2 If different numbers of the relevant species are on both sides, balance them 3 Work out the oxidation number of the element before and after the change 4 Add electrons to one side of the equation so the oxidation numbers balance 5 ...
... 1 Work out the formula of the species before and after the change; 2 If different numbers of the relevant species are on both sides, balance them 3 Work out the oxidation number of the element before and after the change 4 Add electrons to one side of the equation so the oxidation numbers balance 5 ...
Organic Chemistry II / CHEM 252 Chapter 21 – Phenoles and Aryl
... – Formally this results in removal of a pair of electrons and two protons from hydroquinone - This reaction is reversible ...
... – Formally this results in removal of a pair of electrons and two protons from hydroquinone - This reaction is reversible ...
Multiphoton ionization of inner-valence electrons and fragmentation
... For the MPI of ethylene the value of gcr is calculated to be ; 3.05. Our experimental result on the MPI of rare gas atoms w13x has shown that the criterion expressed by Eq. Ž8. is too strong and in practice the criterion g - gcr is good enough for real experiments. It is impossible to give a more pr ...
... For the MPI of ethylene the value of gcr is calculated to be ; 3.05. Our experimental result on the MPI of rare gas atoms w13x has shown that the criterion expressed by Eq. Ž8. is too strong and in practice the criterion g - gcr is good enough for real experiments. It is impossible to give a more pr ...
Ch 25 Hydrocarbon Compounds
... The cooling system of a small van contains 6690 grams of 1,2ethanediol. Some properties of water and 1,2-ethanediol are given in the table below. ...
... The cooling system of a small van contains 6690 grams of 1,2ethanediol. Some properties of water and 1,2-ethanediol are given in the table below. ...
Spring 2015 CH 421 Name ________________________________________ Section ___________ Post‐lab 3: The Grignard Reaction: Preparation of an Alcohol
... 7) Esters undergo reaction with a Grignard reagent to form an intermediate ketone, which then undergoes further attack by a second equivalent of the Grignard reagent. Indicate the ester starting material and the Grignard reagent that would provide the following alcohol after aqueou ...
... 7) Esters undergo reaction with a Grignard reagent to form an intermediate ketone, which then undergoes further attack by a second equivalent of the Grignard reagent. Indicate the ester starting material and the Grignard reagent that would provide the following alcohol after aqueou ...
CH 106 - Clackamas Community College
... Describe how amide bonds (or peptide linkages) are formed and how they can be broken. Recognize an asymmetric (chiral) carbon atom in the formula of a compound. Recognize heterocyclic compounds given their structural formulas. Distinguish between aromatic, polycyclic, and heterocyclic compounds. Ide ...
... Describe how amide bonds (or peptide linkages) are formed and how they can be broken. Recognize an asymmetric (chiral) carbon atom in the formula of a compound. Recognize heterocyclic compounds given their structural formulas. Distinguish between aromatic, polycyclic, and heterocyclic compounds. Ide ...
OCR A Level Chemistry B (Salters) Multiple Choice Questions Quiz
... with either molecule (both could react with bromine in the presence of an acid catalyst). The learner is trying to distinguish between saturated and unsaturated compounds but in both molecules the double bond is a C=O not C=C. ...
... with either molecule (both could react with bromine in the presence of an acid catalyst). The learner is trying to distinguish between saturated and unsaturated compounds but in both molecules the double bond is a C=O not C=C. ...
File - Mr Weng`s IB Chemistry
... In the reaction between methane and chlorine either can be used, however... In the laboratory a source of UV light (or sunlight) is favoured. ...
... In the reaction between methane and chlorine either can be used, however... In the laboratory a source of UV light (or sunlight) is favoured. ...
Chapter 5
... group as the parent alkane and number it from the end that gives the -OH the lower number. 2. Change the ending of the parent alkane from -e to -ol and use a number to show the location of the -OH group; for cyclic alcohols, the carbon bearing the -OH group is carbon1. 3. Name and number substituent ...
... group as the parent alkane and number it from the end that gives the -OH the lower number. 2. Change the ending of the parent alkane from -e to -ol and use a number to show the location of the -OH group; for cyclic alcohols, the carbon bearing the -OH group is carbon1. 3. Name and number substituent ...
course outline - Clackamas Community College
... Recognize amines, amides, and amino acids (α-amino acids in particular) from their formulas. Name simple amines, amides, and amino acids given the formulas. Draw structural formulas for simple amines and amides given the names. Describe how nitrogen-containing molecules can participate in hy ...
... Recognize amines, amides, and amino acids (α-amino acids in particular) from their formulas. Name simple amines, amides, and amino acids given the formulas. Draw structural formulas for simple amines and amides given the names. Describe how nitrogen-containing molecules can participate in hy ...
MOLECULAR REPRESENTATIONS AND INFRARED
... C=O: 1630-1780 cm-1, strong absorption If you need to use other frequencies to identify other functional groups (and sometimes you will), a table of IR frequencies will be provided. ...
... C=O: 1630-1780 cm-1, strong absorption If you need to use other frequencies to identify other functional groups (and sometimes you will), a table of IR frequencies will be provided. ...
1. 4-methyl-4-octanol oxidizes to form a) 4-methyl-4
... 7. Which of the following will have the highest boiling point? a) 2-hexanone b) 2-hexanol c) hexanal d) hexane 8. What is the name of the reaction between an alcohol and an aldehyde? a) oxidation b) reduction c) addition d) none of the above 9. What determines if a molecule is a reducing sugar? a) ...
... 7. Which of the following will have the highest boiling point? a) 2-hexanone b) 2-hexanol c) hexanal d) hexane 8. What is the name of the reaction between an alcohol and an aldehyde? a) oxidation b) reduction c) addition d) none of the above 9. What determines if a molecule is a reducing sugar? a) ...
Chapter
... Types of Formula Structural Formula • Structural Formula describe the kinds of elements found in the compound, the numbers of their atoms, order of atom attachment, and the kind of attachment they do not directly describe the 3-dimensional shape, but an experienced chemist can make a good guess a ...
... Types of Formula Structural Formula • Structural Formula describe the kinds of elements found in the compound, the numbers of their atoms, order of atom attachment, and the kind of attachment they do not directly describe the 3-dimensional shape, but an experienced chemist can make a good guess a ...
OXIDATION NUMBERS
... 2 If different numbers of the relevant species are on both sides, balance them 3 Work out the oxidation number of the element before and after the change 4 Add electrons to one side of the equation so the oxidation numbers balance 5 If the charges on all the species (ions and electrons) on either si ...
... 2 If different numbers of the relevant species are on both sides, balance them 3 Work out the oxidation number of the element before and after the change 4 Add electrons to one side of the equation so the oxidation numbers balance 5 If the charges on all the species (ions and electrons) on either si ...
Homoaromaticity

Homoaromaticity in organic chemistry refers to a special case of aromaticity in which conjugation is interrupted by a single sp3 hybridized carbon atom. Although this sp3 center disrupts the continuous overlap of p-orbitals, traditionally thought to be a requirement for aromaticity, considerable thermodynamic stability and many of the spectroscopic, magnetic, and chemical properties associated with aromatic compounds are still observed for such compounds. This formal discontinuity is apparently bridged by p-orbital overlap, maintaining a contiguous cycle of π electrons that is responsible for this preserved chemical stability.The concept of homoaromaticity was pioneered by Saul Winstein in 1959, prompted by his studies of the “tris-homocyclopropenyl” cation. Since the publication of Winstein's paper, much research has been devoted to understanding and classifying these molecules, which represent an additional “class” of aromatic molecules included under the continuously broadening definition of aromaticity. To date, homoaromatic compounds are known to exist as cationic and anionic species, and some studies support the existence of neutral homoaromatic molecules, though these are less common. The 'homotropylium' cation (C8H9+) is perhaps the best studied example of a homoaromatic compound.