plumbum thiogallate optical properties
... placed. The wafers were oriented so that they did not change the condition of polarization of incident radiation. Typical number of ob ctory optical quality). A decrease in the number of observed rings is connected with phase distortions introduced by the wafer into the stopped beam of radiation of ...
... placed. The wafers were oriented so that they did not change the condition of polarization of incident radiation. Typical number of ob ctory optical quality). A decrease in the number of observed rings is connected with phase distortions introduced by the wafer into the stopped beam of radiation of ...
BioN02 Introduction to organic chemistry Summer 2014
... The smaller of the two numbers designating the carbon atoms of the double bond is used as the double bond locator. If more than one double bond is present the compound is named as a diene, triene or equivalent prefix indicating the number of double bonds, and each double bond is assigned a locator n ...
... The smaller of the two numbers designating the carbon atoms of the double bond is used as the double bond locator. If more than one double bond is present the compound is named as a diene, triene or equivalent prefix indicating the number of double bonds, and each double bond is assigned a locator n ...
Answers, Problem Set 12 (full)... “2,4
... derivatives, generating as many as you can with each of those before moving on. There is one heptane derivative, two hexane derivatives, five pentane derivatives, and one butane derivative (see below). Add the names to the after you generate the structures. Tip #2: Even if you are not asked for the ...
... derivatives, generating as many as you can with each of those before moving on. There is one heptane derivative, two hexane derivatives, five pentane derivatives, and one butane derivative (see below). Add the names to the after you generate the structures. Tip #2: Even if you are not asked for the ...
Life Chemistry and Energy
... polar molecule through hydrogen bonds. Hydrophilic (“water-loving”)—in aqueous solutions, polar molecules become separated and surrounded by water molecules Nonpolar molecules are called hydrophobic (“water-hating”); the interactions between them are hydrophobic interactions. ...
... polar molecule through hydrogen bonds. Hydrophilic (“water-loving”)—in aqueous solutions, polar molecules become separated and surrounded by water molecules Nonpolar molecules are called hydrophobic (“water-hating”); the interactions between them are hydrophobic interactions. ...
Lecture Presentation to accompany Principles of Life
... polar molecule through hydrogen bonds. Hydrophilic (“water-loving”)—in aqueous solutions, polar molecules become separated and surrounded by water molecules Nonpolar molecules are called hydrophobic (“water-hating”); the interactions between them are hydrophobic interactions. ...
... polar molecule through hydrogen bonds. Hydrophilic (“water-loving”)—in aqueous solutions, polar molecules become separated and surrounded by water molecules Nonpolar molecules are called hydrophobic (“water-hating”); the interactions between them are hydrophobic interactions. ...
Chapter 7: Alkenes and Alkynes – Properties and Synthesis
... Ch 8.16–8.21: Oxidation of alkenes, 1,2-Diols(glycols), syn-dihydoxylation ...
... Ch 8.16–8.21: Oxidation of alkenes, 1,2-Diols(glycols), syn-dihydoxylation ...
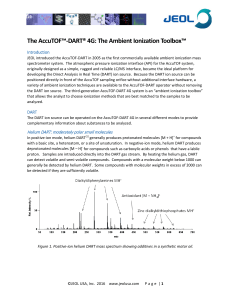
The Ambient Ionization Toolbox
... AccuTOF atmospheric pressure interface while the DART is operated in negative-ion mode as a source of O2. Rapid expansion into vacuum cools the weakly bound [M + O2] ─• species for detection by the AccuTOF. ...
... AccuTOF atmospheric pressure interface while the DART is operated in negative-ion mode as a source of O2. Rapid expansion into vacuum cools the weakly bound [M + O2] ─• species for detection by the AccuTOF. ...
Ch02 Lecture-Life Chemistry and Energy (1)
... polar molecule through hydrogen bonds. Hydrophilic (“water-loving”)—in aqueous solutions, polar molecules become separated and surrounded by water molecules Nonpolar molecules are called hydrophobic (“water-hating”); the interactions between them are hydrophobic interactions. ...
... polar molecule through hydrogen bonds. Hydrophilic (“water-loving”)—in aqueous solutions, polar molecules become separated and surrounded by water molecules Nonpolar molecules are called hydrophobic (“water-hating”); the interactions between them are hydrophobic interactions. ...
2B Synthesis Organic synthesis of aliphatic and aromatic
... The chiral carbons often have an asterisk on to show the chiral centre. Compounds that do not have 4 different groups around a carbon atom are said to be achiral. (most compounds are these). The 2 isomers are called enantiomers. You must draw them in 3D and a mirror line is often drawn to help with ...
... The chiral carbons often have an asterisk on to show the chiral centre. Compounds that do not have 4 different groups around a carbon atom are said to be achiral. (most compounds are these). The 2 isomers are called enantiomers. You must draw them in 3D and a mirror line is often drawn to help with ...
2013 Final Exam File - Fiji National University
... (b) Draw the eclipsed and staggered conformations of 2-methylpropane ( (CH3)3CH ), as Newman projections, with respect to rotation about any one of the C–C bonds. [8 marks] (c) Explain what is meant by each of the following terms. (i) Meso compound. (ii) Enantiomers. [8 marks] (d) The mass spectrum ...
... (b) Draw the eclipsed and staggered conformations of 2-methylpropane ( (CH3)3CH ), as Newman projections, with respect to rotation about any one of the C–C bonds. [8 marks] (c) Explain what is meant by each of the following terms. (i) Meso compound. (ii) Enantiomers. [8 marks] (d) The mass spectrum ...
Experiment 4 - Macalester College
... Molecules with bonding and nonbonding electrons on their central atoms are susceptible to distortions (i.e., non-ideal bond angles). Let’s investigate some of the features that lead to distortions. Our goal is not only to rationalize why molecules possess distortions, but ultimately to estimate dist ...
... Molecules with bonding and nonbonding electrons on their central atoms are susceptible to distortions (i.e., non-ideal bond angles). Let’s investigate some of the features that lead to distortions. Our goal is not only to rationalize why molecules possess distortions, but ultimately to estimate dist ...
Carbonyl compounds
... so that the two homologous series are more conveniently considered together. However, the attachment of a hydrogen to the carbonyl group of an aldehyde does give it certain properties which ketones do not share, and which enables the two families of organic compounds to be distinguished from one ano ...
... so that the two homologous series are more conveniently considered together. However, the attachment of a hydrogen to the carbonyl group of an aldehyde does give it certain properties which ketones do not share, and which enables the two families of organic compounds to be distinguished from one ano ...
AP Chemistry - West Bloomfield School District
... The other metals in the ore do not react with carbon monoxide. If 94.2 g of a metal mixture produced 98.4 g of Ni(CO) 4 , what is the mass percent of nickel in the original sample? **The following are new problems. ☺ (SEE “Unknown CH/CHO Assistance Sheet”, and Sample Problem at end of packet) 69. Al ...
... The other metals in the ore do not react with carbon monoxide. If 94.2 g of a metal mixture produced 98.4 g of Ni(CO) 4 , what is the mass percent of nickel in the original sample? **The following are new problems. ☺ (SEE “Unknown CH/CHO Assistance Sheet”, and Sample Problem at end of packet) 69. Al ...
Aldehydes and Ketones
... In general, simple alcohols like methanol and ethanol are not used in the formation of acetals (particularly from less-reactive ketones!) The main reason is entropy - you’ve got to get three molecules together to form one - that’s not so good! The very common way around this is to use a glycol - eth ...
... In general, simple alcohols like methanol and ethanol are not used in the formation of acetals (particularly from less-reactive ketones!) The main reason is entropy - you’ve got to get three molecules together to form one - that’s not so good! The very common way around this is to use a glycol - eth ...
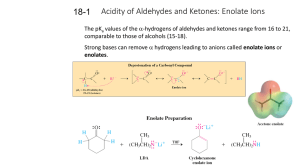
18-1 Enolates (PPT)
... enol form is less stable by 8-12 kcal mol-1. However, for acetaldehyde, the enol form is about 100-times more stable than that of acetone because the less substituted aldehyde carbonyl is more stable than the more substituted ketone carbonyl. ...
... enol form is less stable by 8-12 kcal mol-1. However, for acetaldehyde, the enol form is about 100-times more stable than that of acetone because the less substituted aldehyde carbonyl is more stable than the more substituted ketone carbonyl. ...
Chem 150 Unit 2 - Hydrocarbons & Functional Groups
... • Note how the boiling points increase with molecular weight. ...
... • Note how the boiling points increase with molecular weight. ...
Compounds Containing A Single Bond To A
... • An alkyl halide is named as an alkane with a halogen substituent— ...
... • An alkyl halide is named as an alkane with a halogen substituent— ...
Alkenes
... Compound (a) is stable. Although the double bond is at a bridgehead, it is not a bridged bicyclic system. The trans double bond is in a ten membered ring. Compound (b) is a Bredt’s rule violation and is not stable. The largest ring contains six carbon atoms, and the trans double bond cannot be stabl ...
... Compound (a) is stable. Although the double bond is at a bridgehead, it is not a bridged bicyclic system. The trans double bond is in a ten membered ring. Compound (b) is a Bredt’s rule violation and is not stable. The largest ring contains six carbon atoms, and the trans double bond cannot be stabl ...
...detail
... of dipole moment and its application in aliphatic and aromatic compounds. Inter- and intramolecular forces and their effects on physical and chemical properties of molecules. (6 lectures) 5. Homolysis and heterolysis of bonds: generation, structure and reactivity of carbocations (carbenium and carbo ...
... of dipole moment and its application in aliphatic and aromatic compounds. Inter- and intramolecular forces and their effects on physical and chemical properties of molecules. (6 lectures) 5. Homolysis and heterolysis of bonds: generation, structure and reactivity of carbocations (carbenium and carbo ...
II. Main types of organometallic compounds
... The octet rule is a chemical rule of thumb that states that atoms of main-group elements tend to combine in such a way that each atom has eight electrons in its valence shell, the same electronic configuration as a noble gas. The rule is especially applicable to carbon, nitrogen, oxygen, and the hal ...
... The octet rule is a chemical rule of thumb that states that atoms of main-group elements tend to combine in such a way that each atom has eight electrons in its valence shell, the same electronic configuration as a noble gas. The rule is especially applicable to carbon, nitrogen, oxygen, and the hal ...
Lecture #
... This is a list of topics we will be covering to help you in preparation for exams. Topics from Clayden are indicated clearly by chapter and page numbers where necessary. Topics NOT from Clayden are listed in italics. PLTL topics are in CAPS. This document will be updated throughout the term. The goa ...
... This is a list of topics we will be covering to help you in preparation for exams. Topics from Clayden are indicated clearly by chapter and page numbers where necessary. Topics NOT from Clayden are listed in italics. PLTL topics are in CAPS. This document will be updated throughout the term. The goa ...
Homoaromaticity

Homoaromaticity in organic chemistry refers to a special case of aromaticity in which conjugation is interrupted by a single sp3 hybridized carbon atom. Although this sp3 center disrupts the continuous overlap of p-orbitals, traditionally thought to be a requirement for aromaticity, considerable thermodynamic stability and many of the spectroscopic, magnetic, and chemical properties associated with aromatic compounds are still observed for such compounds. This formal discontinuity is apparently bridged by p-orbital overlap, maintaining a contiguous cycle of π electrons that is responsible for this preserved chemical stability.The concept of homoaromaticity was pioneered by Saul Winstein in 1959, prompted by his studies of the “tris-homocyclopropenyl” cation. Since the publication of Winstein's paper, much research has been devoted to understanding and classifying these molecules, which represent an additional “class” of aromatic molecules included under the continuously broadening definition of aromaticity. To date, homoaromatic compounds are known to exist as cationic and anionic species, and some studies support the existence of neutral homoaromatic molecules, though these are less common. The 'homotropylium' cation (C8H9+) is perhaps the best studied example of a homoaromatic compound.