Mr. Kent`s Organic Chemistry Unit Notes B. ______ will not only
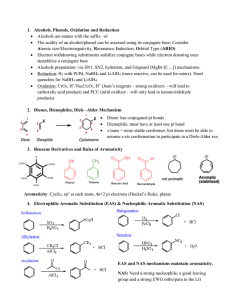
... Rule #5- When there are 2 different side chains name them in alphabetical order using the carbon prefix (meth, eth..). ...
... Rule #5- When there are 2 different side chains name them in alphabetical order using the carbon prefix (meth, eth..). ...
Test3
... 3. When HCl is added to pure water, HCl molecules lose protons, while water molecules gain protons. In this reaction, HCl is a(n) a. b. c. d. ...
... 3. When HCl is added to pure water, HCl molecules lose protons, while water molecules gain protons. In this reaction, HCl is a(n) a. b. c. d. ...
How Ionic Bonds Form?
... • Energy holds the electrons in orbit around the nucleus • Energy is needed to remove valence electrons from one atom and attach them to another • These ionic bonds are very strong and difficult to break – for example, try burning salt • These compounds have high melting and boiling points and are g ...
... • Energy holds the electrons in orbit around the nucleus • Energy is needed to remove valence electrons from one atom and attach them to another • These ionic bonds are very strong and difficult to break – for example, try burning salt • These compounds have high melting and boiling points and are g ...
Aromatic Compounds
... • Structure must be cyclic with conjugated pi bonds. • Each atom in the ring must have an unhybridized p orbital. • The p orbitals must overlap continuously around the ring. (Usually planar structure) • Compound is more stable than its openchain counterpart. ...
... • Structure must be cyclic with conjugated pi bonds. • Each atom in the ring must have an unhybridized p orbital. • The p orbitals must overlap continuously around the ring. (Usually planar structure) • Compound is more stable than its openchain counterpart. ...
Organic Chemistry
... What does it mean to be organic? To be an organic compound means that you contain carbon ...
... What does it mean to be organic? To be an organic compound means that you contain carbon ...
Chem 400 Review Chem 350 JJ.S17
... assume s-cis conformation to participate in a Diels-Alder rxn ...
... assume s-cis conformation to participate in a Diels-Alder rxn ...
Lecture 3 Chemistry
... Atoms also have Electrons, negatively charged subatomic particles Arrangement Two dimensionally vs 3-D 1st shell - 2 electrons 2nd Shell - 8 Electrons 3rd Shell - 8 Electrons 4th Shell - 18 Electrons Outer shell 8 electrons *** Octet Number of electrons in outer shell determines bonding properties c ...
... Atoms also have Electrons, negatively charged subatomic particles Arrangement Two dimensionally vs 3-D 1st shell - 2 electrons 2nd Shell - 8 Electrons 3rd Shell - 8 Electrons 4th Shell - 18 Electrons Outer shell 8 electrons *** Octet Number of electrons in outer shell determines bonding properties c ...
Name
... explain how many atoms are in that compound. Name each atom and write how many there are next to it. See the example, which is done for you. Substance (Common Name) ...
... explain how many atoms are in that compound. Name each atom and write how many there are next to it. See the example, which is done for you. Substance (Common Name) ...
Chemical bond - Physical Science
... Atomic Stability • All elements want to reach stability • An atom is considered chemically stable when its outermost energy level has the maximum number of electrons ▫ Ex: hydrogen and helium are stable with two electrons ▫ Ex: the outer energy levels of all other elements are stable when they cont ...
... Atomic Stability • All elements want to reach stability • An atom is considered chemically stable when its outermost energy level has the maximum number of electrons ▫ Ex: hydrogen and helium are stable with two electrons ▫ Ex: the outer energy levels of all other elements are stable when they cont ...
Ionic and Covalent bonding (WLC)
... • Shows an alloy mixture. It is NOT a compound but a physical mixing of a metal plus at least one other material (shown by red circle, it can be another metal eg Ni, a non-metal eg C or a compound of carbon or manganese, and it can be bigger or smaller than iron atoms). Many alloys are produced to g ...
... • Shows an alloy mixture. It is NOT a compound but a physical mixing of a metal plus at least one other material (shown by red circle, it can be another metal eg Ni, a non-metal eg C or a compound of carbon or manganese, and it can be bigger or smaller than iron atoms). Many alloys are produced to g ...
Slide 1
... phosphorus atom is bonded to four oxygen atoms; one oxygen is bonded to the carbon skeleton; two oxygens carry negative charges. The phosphate group (—OPO32–, abbreviated P ) is an ionized form of a phosphoric acid group (—OPO3H2; note the two ...
... phosphorus atom is bonded to four oxygen atoms; one oxygen is bonded to the carbon skeleton; two oxygens carry negative charges. The phosphate group (—OPO32–, abbreviated P ) is an ionized form of a phosphoric acid group (—OPO3H2; note the two ...
Unit C
... formulas, using International Union of Pure and Applied Chemistry (IUPAC) nomenclature guidelines, for saturated and unsaturated aliphatic (including cyclic) and aromatic carbon compounds • containing up to 10 carbon atoms in the parent chain (e.g., pentane; 3ethyl-2,4- dimethylpentane) or cyclic st ...
... formulas, using International Union of Pure and Applied Chemistry (IUPAC) nomenclature guidelines, for saturated and unsaturated aliphatic (including cyclic) and aromatic carbon compounds • containing up to 10 carbon atoms in the parent chain (e.g., pentane; 3ethyl-2,4- dimethylpentane) or cyclic st ...
1 up to alkynes (4 days)
... Carbon is the element necessary to classify a compound as organic . . . One reason that carbon has its own branch of chemistry is that there are over 10 million known carbon compounds, and about 90% of new compounds synthesized each year contain carbon. ...
... Carbon is the element necessary to classify a compound as organic . . . One reason that carbon has its own branch of chemistry is that there are over 10 million known carbon compounds, and about 90% of new compounds synthesized each year contain carbon. ...
Ch.04Carbon and the Molecular Diversity of Life
... phosphorus atom is bonded to four oxygen atoms; one oxygen is bonded to the carbon skeleton; two oxygens carry negative charges. The phosphate group ...
... phosphorus atom is bonded to four oxygen atoms; one oxygen is bonded to the carbon skeleton; two oxygens carry negative charges. The phosphate group ...
13.26 SUMMARY
... and one double bond and an index of hydrogen deficiency of 2. Oxygen atoms have no effect on the index of hydrogen deficiency. A halogen substituent, like hydrogen, is monovalent and when present in a molecular formula is treated as if it were hydrogen for counting purposes. How does one distinguish ...
... and one double bond and an index of hydrogen deficiency of 2. Oxygen atoms have no effect on the index of hydrogen deficiency. A halogen substituent, like hydrogen, is monovalent and when present in a molecular formula is treated as if it were hydrogen for counting purposes. How does one distinguish ...
World of Chemistry Chapter 20—Organic Chemistry
... B. Carbon forms strong bonds to itself and to many other elements C. More than any other element, carbon can form long chains of molecules II. Section 20.2—Alkanes A. Alkanes are saturated hydrocarbons—which means that they are a chain of carbon and hydrogen atoms where each carbon atom is bound to ...
... B. Carbon forms strong bonds to itself and to many other elements C. More than any other element, carbon can form long chains of molecules II. Section 20.2—Alkanes A. Alkanes are saturated hydrocarbons—which means that they are a chain of carbon and hydrogen atoms where each carbon atom is bound to ...
Atoms and the Periodic Table
... A negatively charged atom is called an Anion – it has more electrons than protons. ...
... A negatively charged atom is called an Anion – it has more electrons than protons. ...
File
... • In Organic Chemistry, you will only be using the prefixes for one to ten. All prefixes have the ending of “yl” but is removed for specific families/groups. Details later. • 1 – meth 6 - hex • 2 – eth 7 - hept • 3 – prop 8 - oct • 4 – but 9 - non • 5 – pent 10 - dec ...
... • In Organic Chemistry, you will only be using the prefixes for one to ten. All prefixes have the ending of “yl” but is removed for specific families/groups. Details later. • 1 – meth 6 - hex • 2 – eth 7 - hept • 3 – prop 8 - oct • 4 – but 9 - non • 5 – pent 10 - dec ...
a. Rank by acidity. The most acidic compound is 1, wh
... You may use any inorganic reagent you desire and any organic compound which contains four carbons or less. (15 points each) a. HC CH ...
... You may use any inorganic reagent you desire and any organic compound which contains four carbons or less. (15 points each) a. HC CH ...
AP Ch. 25 Notes
... - sp3 hybridized carbons for tetrahedral geometries - sp2 hybridized carbons for trigonal planar geometries - sp hybridized carbons for linear geometries ...
... - sp3 hybridized carbons for tetrahedral geometries - sp2 hybridized carbons for trigonal planar geometries - sp hybridized carbons for linear geometries ...
Homoaromaticity

Homoaromaticity in organic chemistry refers to a special case of aromaticity in which conjugation is interrupted by a single sp3 hybridized carbon atom. Although this sp3 center disrupts the continuous overlap of p-orbitals, traditionally thought to be a requirement for aromaticity, considerable thermodynamic stability and many of the spectroscopic, magnetic, and chemical properties associated with aromatic compounds are still observed for such compounds. This formal discontinuity is apparently bridged by p-orbital overlap, maintaining a contiguous cycle of π electrons that is responsible for this preserved chemical stability.The concept of homoaromaticity was pioneered by Saul Winstein in 1959, prompted by his studies of the “tris-homocyclopropenyl” cation. Since the publication of Winstein's paper, much research has been devoted to understanding and classifying these molecules, which represent an additional “class” of aromatic molecules included under the continuously broadening definition of aromaticity. To date, homoaromatic compounds are known to exist as cationic and anionic species, and some studies support the existence of neutral homoaromatic molecules, though these are less common. The 'homotropylium' cation (C8H9+) is perhaps the best studied example of a homoaromatic compound.