South Pasadena • Chemistry Name Period Date 3 · Organic
... o Demonstrate that double and triple bonds cannot rotate like a single bond. o State that “saturated” means “saturated with hydrogens” and describes alkanes. State that alkenes, alkynes, and cyclic hydrocarbons are all “unsaturated.” ...
... o Demonstrate that double and triple bonds cannot rotate like a single bond. o State that “saturated” means “saturated with hydrogens” and describes alkanes. State that alkenes, alkynes, and cyclic hydrocarbons are all “unsaturated.” ...
Chem 30CL-Lecture 15..
... • Acetylene, carbon monoxide and alcohols are reacted in the presence of a catalyst like Ni(CO)4, HCo(CO)4 or Fe(CO)5 to yield acrylic acid esters • If water is used instead of alcohols, the carboxylic acid is obtained (i.e., acrylic acid) • The BHC process to synthesize of ibuprofen uses a palladiu ...
... • Acetylene, carbon monoxide and alcohols are reacted in the presence of a catalyst like Ni(CO)4, HCo(CO)4 or Fe(CO)5 to yield acrylic acid esters • If water is used instead of alcohols, the carboxylic acid is obtained (i.e., acrylic acid) • The BHC process to synthesize of ibuprofen uses a palladiu ...
Chapter 2 PPT - Richsingiser.com
... • Often the same elements form more than one compound. Numerical prefixes are used to give the number of atoms present in the molecule. Number one two three four five six ...
... • Often the same elements form more than one compound. Numerical prefixes are used to give the number of atoms present in the molecule. Number one two three four five six ...
Elements Found in Living Things
... Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds can be classified in two broad categories --- organic and inorganic compounds. Organic compounds are made primarily of carbon. Carbon has four ...
... Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds can be classified in two broad categories --- organic and inorganic compounds. Organic compounds are made primarily of carbon. Carbon has four ...
Functional Groups
... Ethanol is the intoxicating substance in alcoholic beverages. It is a depressant that can be fatal if taken in large doses at once. Denatured alcohol is ethanol with an added substance to make it toxic (poisonous). Denatured alcohol is used as a reactant or as a solvent in ...
... Ethanol is the intoxicating substance in alcoholic beverages. It is a depressant that can be fatal if taken in large doses at once. Denatured alcohol is ethanol with an added substance to make it toxic (poisonous). Denatured alcohol is used as a reactant or as a solvent in ...
The influence of oxidation state on the electronegativity of tin
... tin and the electronegativity of the second moiety. In general, the effective electronegativity of any atom increases with increasing oxidation state. Covalent character is a feature of bonds between systems with both reasonably high and similar electronegativities. In the tetravalent oxidation stat ...
... tin and the electronegativity of the second moiety. In general, the effective electronegativity of any atom increases with increasing oxidation state. Covalent character is a feature of bonds between systems with both reasonably high and similar electronegativities. In the tetravalent oxidation stat ...
Intro to Organic Compounds
... because they have two H atoms fewer than alkenes with the same number of carbons. Because a carbon in a C≡C bond can bond to only one other atom, the geometry around each C atom is linear (180°): each C is sp hybridized. Alkynes are named in the same way as alkenes, except that the suffix is -yne. ...
... because they have two H atoms fewer than alkenes with the same number of carbons. Because a carbon in a C≡C bond can bond to only one other atom, the geometry around each C atom is linear (180°): each C is sp hybridized. Alkynes are named in the same way as alkenes, except that the suffix is -yne. ...
8B31A38F-1279-3B00-CDA90244BEA11A7B
... 2. Add prefixes to indicate # of atoms. Omit mono- prefix on the FIRST element. Mono- is OPTIONAL on the SECOND element (in this class, it’s NOT optional!). 3. Change the ending of the second element to -ide. ...
... 2. Add prefixes to indicate # of atoms. Omit mono- prefix on the FIRST element. Mono- is OPTIONAL on the SECOND element (in this class, it’s NOT optional!). 3. Change the ending of the second element to -ide. ...
Chapter 26 Functional Groups and Organic Reactions
... compounds with an -OH group – The -OH functional group in alcohols is called a “hydroxyl” group; thus ROH can represent the formula, where “R” is an alkyl group How is this different from the hydroxide ion? (covalently bonded to the carbonnot ionically as in hydroxides) ...
... compounds with an -OH group – The -OH functional group in alcohols is called a “hydroxyl” group; thus ROH can represent the formula, where “R” is an alkyl group How is this different from the hydroxide ion? (covalently bonded to the carbonnot ionically as in hydroxides) ...
GR.12 ALCOHOL WITH KEY 06-07
... A. Calculate the molar mass of (A), knowing that the gas obtained by the vaporization of 0.74g of this compound occupy, at T = 400oK and P = 1 atm, a volume of 328mL. Given: R = 0.082L.atm.mol-1K-1. B. Show that the molecular formula of (A) is C4H10O. The molar masses of H, C and O in g/mol are 1, 1 ...
... A. Calculate the molar mass of (A), knowing that the gas obtained by the vaporization of 0.74g of this compound occupy, at T = 400oK and P = 1 atm, a volume of 328mL. Given: R = 0.082L.atm.mol-1K-1. B. Show that the molecular formula of (A) is C4H10O. The molar masses of H, C and O in g/mol are 1, 1 ...
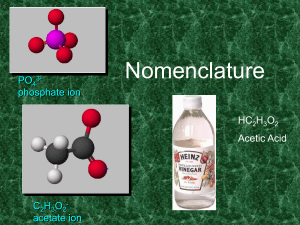
UNIT_3_PART_5[3]
... When the metal involved in the ionic bond has more than one oxidations state/possible charge we need to identify which ion it is. This usually occurs with the transition metals. Any ion that appears on your common ion sheet more than once is one of these types of metals. There are 2 ways to do thi ...
... When the metal involved in the ionic bond has more than one oxidations state/possible charge we need to identify which ion it is. This usually occurs with the transition metals. Any ion that appears on your common ion sheet more than once is one of these types of metals. There are 2 ways to do thi ...
1. Functional groups contribute to the molecular diversity of life
... • The components of organic molecules that are most commonly involved in chemical reactions are known as functional groups. – Functional groups are attachments that replace one or more hydrogen atoms to the carbon skeleton of the hydrocarbon. ...
... • The components of organic molecules that are most commonly involved in chemical reactions are known as functional groups. – Functional groups are attachments that replace one or more hydrogen atoms to the carbon skeleton of the hydrocarbon. ...
Functional Groups
... • The components of organic molecules that are most commonly involved in chemical reactions are known as functional groups. • Functional groups are attachments that replace one or more hydrogen atoms to the carbon skeleton of the hydrocarbon. ...
... • The components of organic molecules that are most commonly involved in chemical reactions are known as functional groups. • Functional groups are attachments that replace one or more hydrogen atoms to the carbon skeleton of the hydrocarbon. ...
functional groups
... • The components of organic molecules that are most commonly involved in chemical reactions are known as functional groups. • Functional groups are attachments that replace one or more hydrogen atoms to the carbon skeleton of the hydrocarbon. ...
... • The components of organic molecules that are most commonly involved in chemical reactions are known as functional groups. • Functional groups are attachments that replace one or more hydrogen atoms to the carbon skeleton of the hydrocarbon. ...
Document
... Changes The products of a chemical reaction may often be predicted by applying known facts about common reaction types ...
... Changes The products of a chemical reaction may often be predicted by applying known facts about common reaction types ...
chemistry_23 - Bonar Law Memorial
... a class of chemical compounds called ethers. You will read about the chemical characteristics of ethers that make them good anesthetics. Alcohols How are alcohols classified and named? • An alcohol is an organic compound with an — OH group. • The —OH functional group in alcohols is called a hydroxyl ...
... a class of chemical compounds called ethers. You will read about the chemical characteristics of ethers that make them good anesthetics. Alcohols How are alcohols classified and named? • An alcohol is an organic compound with an — OH group. • The —OH functional group in alcohols is called a hydroxyl ...
Gr - loyolascience2
... Why is it true that polar substances dissolve in polar solvents and non-polar substances dissolve in non-polar solvents? There are two important tendencies to consider. First, a substance will dissolve at all if the attraction between solute and solvent particles is stronger than the attraction betw ...
... Why is it true that polar substances dissolve in polar solvents and non-polar substances dissolve in non-polar solvents? There are two important tendencies to consider. First, a substance will dissolve at all if the attraction between solute and solvent particles is stronger than the attraction betw ...
Chapter 4 - ScienceToGo
... The electron configuration of carbon gives it covalent compatibility with many different elements ...
... The electron configuration of carbon gives it covalent compatibility with many different elements ...
Nugget
... The Tröger’s base skeleton is a rigid framework containing two chiral nitrogen atoms at bridgehead positions. Under acid catalysis, the ring system undergoes inversion, but two mechanisms for the inversion have been proposed Our primary goal is to use symmetrically substituted chiral Tröger’s bases ...
... The Tröger’s base skeleton is a rigid framework containing two chiral nitrogen atoms at bridgehead positions. Under acid catalysis, the ring system undergoes inversion, but two mechanisms for the inversion have been proposed Our primary goal is to use symmetrically substituted chiral Tröger’s bases ...
Chapter 2: Alkanes Alkanes are molecules comprised of hydrogen
... In the IUPAC system alcohol names are used as parent names instead of using naming chains as alkyl substituents. The "ane" is dropped from the end of the name and "anol" is added. ...
... In the IUPAC system alcohol names are used as parent names instead of using naming chains as alkyl substituents. The "ane" is dropped from the end of the name and "anol" is added. ...
Section 2.6
... • BUT- only the noble gases are found as isolated atoms • The rest exist as molecules or ions ...
... • BUT- only the noble gases are found as isolated atoms • The rest exist as molecules or ions ...
Homoaromaticity

Homoaromaticity in organic chemistry refers to a special case of aromaticity in which conjugation is interrupted by a single sp3 hybridized carbon atom. Although this sp3 center disrupts the continuous overlap of p-orbitals, traditionally thought to be a requirement for aromaticity, considerable thermodynamic stability and many of the spectroscopic, magnetic, and chemical properties associated with aromatic compounds are still observed for such compounds. This formal discontinuity is apparently bridged by p-orbital overlap, maintaining a contiguous cycle of π electrons that is responsible for this preserved chemical stability.The concept of homoaromaticity was pioneered by Saul Winstein in 1959, prompted by his studies of the “tris-homocyclopropenyl” cation. Since the publication of Winstein's paper, much research has been devoted to understanding and classifying these molecules, which represent an additional “class” of aromatic molecules included under the continuously broadening definition of aromaticity. To date, homoaromatic compounds are known to exist as cationic and anionic species, and some studies support the existence of neutral homoaromatic molecules, though these are less common. The 'homotropylium' cation (C8H9+) is perhaps the best studied example of a homoaromatic compound.












![UNIT_3_PART_5[3]](http://s1.studyres.com/store/data/013887813_1-e384d1258759e65beb0bedcc6881c6f9-300x300.png)