A Crash Course In Organic Chemistry
... Addition of a halogen can reduce or eliminate flammability, and can also increase potency. Depending on the halogen, some of these compounds can cause arrhythmias and/or renal or hepatic toxicity. Compounds containing only bromine are generally not useful. Compounds containing only chlorine are subj ...
... Addition of a halogen can reduce or eliminate flammability, and can also increase potency. Depending on the halogen, some of these compounds can cause arrhythmias and/or renal or hepatic toxicity. Compounds containing only bromine are generally not useful. Compounds containing only chlorine are subj ...
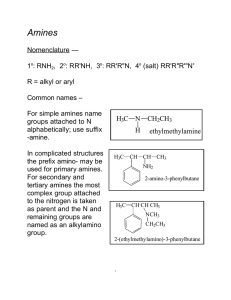
CH3
... 3. Cite some industrial uses of organic compounds ETHER. References: All organic chemistry book will do ...
... 3. Cite some industrial uses of organic compounds ETHER. References: All organic chemistry book will do ...
Full-Text PDF
... During the last three decades, thousands of papers have presented and described the olefin metathesis catalysis, by experimental synthesis and characterization [1], as well as in the validation of computational protocols [2–9]. However, neither a general catalyst for any metathesis reaction [10–12] ...
... During the last three decades, thousands of papers have presented and described the olefin metathesis catalysis, by experimental synthesis and characterization [1], as well as in the validation of computational protocols [2–9]. However, neither a general catalyst for any metathesis reaction [10–12] ...
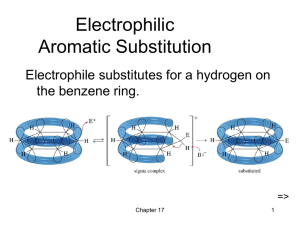
Electrophilic Aromatic Substit
... Activating, O-, PDirecting Substituents • Alkyl groups stabilize the sigma complex by induction, donating electron density through the sigma bond. • Substituents with a lone pair of electrons stabilize the sigma complex by resonance. ...
... Activating, O-, PDirecting Substituents • Alkyl groups stabilize the sigma complex by induction, donating electron density through the sigma bond. • Substituents with a lone pair of electrons stabilize the sigma complex by resonance. ...
study guide spring 2012
... d. CO2 is a gas and carbon is a crystal. ____ 33. In the unbalanced formula equation CO + O2 CO2 + energy, energy a. is absorbed. c. is released. b. can be considered a reactant. d. Both (a) and (b) ____ 34. The word equation for the formula equation shown is C2H5OH + O2 CO2 + H2O + energy a. ca ...
... d. CO2 is a gas and carbon is a crystal. ____ 33. In the unbalanced formula equation CO + O2 CO2 + energy, energy a. is absorbed. c. is released. b. can be considered a reactant. d. Both (a) and (b) ____ 34. The word equation for the formula equation shown is C2H5OH + O2 CO2 + H2O + energy a. ca ...
Slide 1
... The pyridine ring is deactivated towards electrophilic aromatic substitution because of the electronegative nitrogen in the ring. Additionally the non-bonding electron on the nitrogen would react with the electrophile. Reaction only occurs under extreme conditions. Note: That substitution is in the ...
... The pyridine ring is deactivated towards electrophilic aromatic substitution because of the electronegative nitrogen in the ring. Additionally the non-bonding electron on the nitrogen would react with the electrophile. Reaction only occurs under extreme conditions. Note: That substitution is in the ...
Advanced Practical Organic Chemistry
... potassium, manganese, sodium and chlorine, respectively. Molecules with Carbon Most atoms are only capable of forming small molecules. However, one or two can form larger molecules. By far and away the best atom for making large molecules with, is carbon. Carbon can make molecules that have tens, hu ...
... potassium, manganese, sodium and chlorine, respectively. Molecules with Carbon Most atoms are only capable of forming small molecules. However, one or two can form larger molecules. By far and away the best atom for making large molecules with, is carbon. Carbon can make molecules that have tens, hu ...
Synthesis of a TREN in Which the Aryl Substituents are... Atom Macrocycle ̈ller *
... versa. In any case, 8b-2/1 is obviously not a potential ligand of the desired type. The C3-symmetric product (either C3-8b or C3-8b′) could not be purified by crystallization or separated from the small amount of 8b-2/1 remaining in the mixture. However, the C3-symmetric ligand could be characterized ...
... versa. In any case, 8b-2/1 is obviously not a potential ligand of the desired type. The C3-symmetric product (either C3-8b or C3-8b′) could not be purified by crystallization or separated from the small amount of 8b-2/1 remaining in the mixture. However, the C3-symmetric ligand could be characterized ...