Alcohols, etc.
... Ar must be an aromatic ring (eg. Benzene) Thiols have the formula R-SH Disulfides have the formula R-S-S-R R may be aliphatic or aromatic ...
... Ar must be an aromatic ring (eg. Benzene) Thiols have the formula R-SH Disulfides have the formula R-S-S-R R may be aliphatic or aromatic ...
1 Solutions 4a (Chapter 4 problems) Chem151 [Kua]
... (c) The second two parts of this problem involve stoichiometric calculations. The problem gives information about the amounts of both starting materials, so this is a limiting reactant situation. We must calculate the number of moles of each species, construct a table of amounts, and use the result ...
... (c) The second two parts of this problem involve stoichiometric calculations. The problem gives information about the amounts of both starting materials, so this is a limiting reactant situation. We must calculate the number of moles of each species, construct a table of amounts, and use the result ...
Soln Chem 2008Nov(9746)
... that of chemically pure N2, the gas that causes this discrepancy would, therefore, be one of higher mass than N2. [Mr : N2 = 28; Ar = 39.9; He = 4; CH4 = 16; Ne = 20.0] (ans) ...
... that of chemically pure N2, the gas that causes this discrepancy would, therefore, be one of higher mass than N2. [Mr : N2 = 28; Ar = 39.9; He = 4; CH4 = 16; Ne = 20.0] (ans) ...
chapter-15
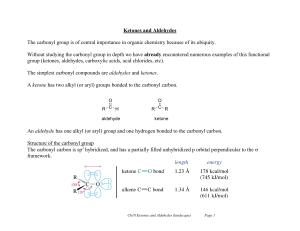
... carbonyl group • for an aldehyde, change the suffix from -e to -al • for an unsaturated aldehyde, show the carbon-carbon double bond by changing the infix from -an- to -en-; the location of the suffix determines the numbering pattern • for a cyclic molecule in which -CHO is bonded to the ring, add t ...
... carbonyl group • for an aldehyde, change the suffix from -e to -al • for an unsaturated aldehyde, show the carbon-carbon double bond by changing the infix from -an- to -en-; the location of the suffix determines the numbering pattern • for a cyclic molecule in which -CHO is bonded to the ring, add t ...
Full-Text PDF
... for epoxidation reactions is well illustrated by some past reviews on the matter. In particular, iron-porphyrins have been the subject of extensive reviews, due to their biological relevance as part of cytochrome P450 [14,15], with their use as catalysts in epoxidation reactions being highlighted on ...
... for epoxidation reactions is well illustrated by some past reviews on the matter. In particular, iron-porphyrins have been the subject of extensive reviews, due to their biological relevance as part of cytochrome P450 [14,15], with their use as catalysts in epoxidation reactions being highlighted on ...
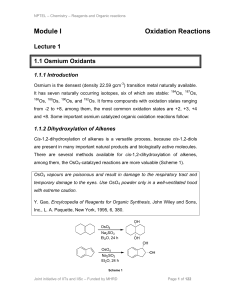
Module I Oxidation Reactions
... among them, the OsO4-catalzyed reactions are more valuable (Scheme 1). OsO4 vapours are poisonous and result in damage to the respiratory tract and temporary damage to the eyes. Use OsO4 powder only in a well-ventilated hood with extreme caution. Y. Gao, Encylcopedia of Reagents for Organic Synthesi ...
... among them, the OsO4-catalzyed reactions are more valuable (Scheme 1). OsO4 vapours are poisonous and result in damage to the respiratory tract and temporary damage to the eyes. Use OsO4 powder only in a well-ventilated hood with extreme caution. Y. Gao, Encylcopedia of Reagents for Organic Synthesi ...
Chapter 12
... If we had the specified amount of all ingredients listed, could we make 4 dozen cookies? What if we had 6 eggs and twice as much of everything else, could we make 9 dozen cookies? What if we only had one egg, could we make 3 dozen cookies? ...
... If we had the specified amount of all ingredients listed, could we make 4 dozen cookies? What if we had 6 eggs and twice as much of everything else, could we make 9 dozen cookies? What if we only had one egg, could we make 3 dozen cookies? ...
Aldehydes/Ketones Solutions
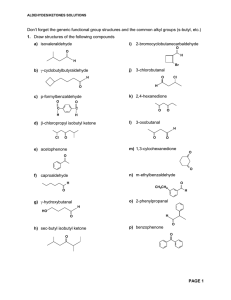
... 6. Write equations showing how the following transformations can be carried out. No mechanisms required but show all reagents and intermediate products formed. More than one step may be necessary a) O O C H ...
... 6. Write equations showing how the following transformations can be carried out. No mechanisms required but show all reagents and intermediate products formed. More than one step may be necessary a) O O C H ...
Reduction of CuO and Cu2O with H2: H Embedding
... were collected in the temperature range 150-300 °C at the beamline X7B (λ ) 0.9034 Å) of the National Synchrotron Light Source (NSLS) in Brookhaven National Laboratory (BNL) using a MAR345 area detector. The XRD diffraction data with a high-order wave vector Q (≈11.5 Å-1) were obtained at the beamli ...
... were collected in the temperature range 150-300 °C at the beamline X7B (λ ) 0.9034 Å) of the National Synchrotron Light Source (NSLS) in Brookhaven National Laboratory (BNL) using a MAR345 area detector. The XRD diffraction data with a high-order wave vector Q (≈11.5 Å-1) were obtained at the beamli ...
LIPIDS
... Structure Lipids are made of 3 fatty acids and glycerol. Glycerol is an alcohol containing 3 hydroxyl groups (OH) . ...
... Structure Lipids are made of 3 fatty acids and glycerol. Glycerol is an alcohol containing 3 hydroxyl groups (OH) . ...




![1 Solutions 4a (Chapter 4 problems) Chem151 [Kua]](http://s1.studyres.com/store/data/002731518_1-574ec10e88e667508364281b6325aeef-300x300.png)