PowerPoint - Naming Hydrocarbons
... • There are 3 main classes of hydrocarbons – Alkanes: Since there are only single bonds throughout these molecules, they are referred to as saturated hydrocarbons. The general formula of an alkane is CnH2n + 2. – Alkenes: An alkene has at least one double bond. It will have 2 less hydrogens (than a ...
... • There are 3 main classes of hydrocarbons – Alkanes: Since there are only single bonds throughout these molecules, they are referred to as saturated hydrocarbons. The general formula of an alkane is CnH2n + 2. – Alkenes: An alkene has at least one double bond. It will have 2 less hydrogens (than a ...
ORGANIC CHEMISTRY
... amine. As usual, numbers are used to indicate the carbon to which the amine substituent is attached. The functional group takes priority over the alkyl group. (In this course we will only have to name primary amines). CH3-NH2 ...
... amine. As usual, numbers are used to indicate the carbon to which the amine substituent is attached. The functional group takes priority over the alkyl group. (In this course we will only have to name primary amines). CH3-NH2 ...
doc
... The characteristics of organic compounds (boiling point, odour, reactivity etc.) depend on the composition and arrangement of atoms. For example the properties of alkanes depend greatly on the number of carbon atoms in the hydrocarbon chain due to the increased strength of the van der Waal attractio ...
... The characteristics of organic compounds (boiling point, odour, reactivity etc.) depend on the composition and arrangement of atoms. For example the properties of alkanes depend greatly on the number of carbon atoms in the hydrocarbon chain due to the increased strength of the van der Waal attractio ...
Compounds with Oxygen Atoms
... • like diethyl ether CH3─CH2─O─CH2─CH3 were used for over a century, but caused nausea and were flammable. • developed by the 1960’s were nonflammable. ...
... • like diethyl ether CH3─CH2─O─CH2─CH3 were used for over a century, but caused nausea and were flammable. • developed by the 1960’s were nonflammable. ...
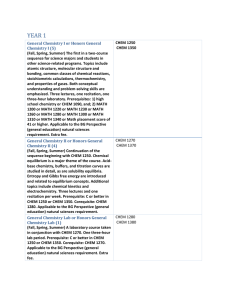
YEAR 1
... three-dimensional structure (stereochemistry), electron-pushing mechanisms, electron delocalization, molecular orbital theory and modern instrumental techniques. Laboratory experiences include chemical separations, examination of reaction selectivity, and isolation of natural products. Prerequisites ...
... three-dimensional structure (stereochemistry), electron-pushing mechanisms, electron delocalization, molecular orbital theory and modern instrumental techniques. Laboratory experiences include chemical separations, examination of reaction selectivity, and isolation of natural products. Prerequisites ...
Unit X Organic Chem (SmartBoard)
... It is possible for two or more molecules to have the same molecular formula (same number and types of atoms) but different arrangement of atoms (structure). All of the following molecules have the molecular formula C5H12. ...
... It is possible for two or more molecules to have the same molecular formula (same number and types of atoms) but different arrangement of atoms (structure). All of the following molecules have the molecular formula C5H12. ...
Chapter 3 Alcohols, Phenols, and Ethers
... Learn the major chemical reaction of alcohols, and learn how to predict the products of dehydration and oxidation reactions. ...
... Learn the major chemical reaction of alcohols, and learn how to predict the products of dehydration and oxidation reactions. ...