Exam 4
... 3) The sum of the masses of two hydrogen atoms (mass number 1) and two neutrons is 4.0330. Why does this differ from the mass of a helium atom (4.0026)? a) Some hydrogen atoms are heavier than others b) The difference is the binding energy of the helium nucleus c) The difference is the experimental ...
... 3) The sum of the masses of two hydrogen atoms (mass number 1) and two neutrons is 4.0330. Why does this differ from the mass of a helium atom (4.0026)? a) Some hydrogen atoms are heavier than others b) The difference is the binding energy of the helium nucleus c) The difference is the experimental ...
CHEMISTRY
... Less E required The more active element replaces the less active one Most active metals (group 1) react w/water and produce metal hydroxides ...
... Less E required The more active element replaces the less active one Most active metals (group 1) react w/water and produce metal hydroxides ...
Chemistry - Vikrama Simhapuri University
... 33) Calamine is the ore of a) Aluminium b) Zinc c) Iron d) Copper 34) How many ions are produced from complex Co(NH3)6Cl2 in solution a) 6 b) 4 c) 3 d) 2 35) During an isothermal expansion of an ideal gas its: a) Internal energy increases b) Enthalpy decreases c) Enthalpy remains unaffected d) Entha ...
... 33) Calamine is the ore of a) Aluminium b) Zinc c) Iron d) Copper 34) How many ions are produced from complex Co(NH3)6Cl2 in solution a) 6 b) 4 c) 3 d) 2 35) During an isothermal expansion of an ideal gas its: a) Internal energy increases b) Enthalpy decreases c) Enthalpy remains unaffected d) Entha ...
Problem Set: Empirical and Molecular Formulas
... 6. Titanium (IV) oxide, TiO2, is used as a pigment in paints and as a whitening and coating agent for paper. It can be made by reacting O2 with TiCl4. TiCl4 + O2 TiO2 + 2 Cl2 (already balanced) a) If 4.5 mol of TiCl4 react with 3.5 mol O2, identify both the limiting and excess reactants. b) How ma ...
... 6. Titanium (IV) oxide, TiO2, is used as a pigment in paints and as a whitening and coating agent for paper. It can be made by reacting O2 with TiCl4. TiCl4 + O2 TiO2 + 2 Cl2 (already balanced) a) If 4.5 mol of TiCl4 react with 3.5 mol O2, identify both the limiting and excess reactants. b) How ma ...
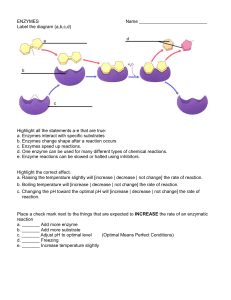
ENZYMES
... a. _______ Add more enzyme b. _______ Add more substrate c. _______ Adjust pH to optimal level (Optimal Means Perfect Conditions) d. _______ Freezing e. _______ Increase temperature slightly ...
... a. _______ Add more enzyme b. _______ Add more substrate c. _______ Adjust pH to optimal level (Optimal Means Perfect Conditions) d. _______ Freezing e. _______ Increase temperature slightly ...
3.4 How do we use the Activity Series
... 3. Will the above reaction take place?_____________________________________________________ 4. Will the reverse reaction take place, Cl2 + 2NaF? __________________________________________ ...
... 3. Will the above reaction take place?_____________________________________________________ 4. Will the reverse reaction take place, Cl2 + 2NaF? __________________________________________ ...
exo and endo experiments
... lost nor gained in chemical reactions, it states that it simply changes form. For that reason, if you had a certain number of atoms of a certain element on the left side of a chemical reaction equation, then you would have to have the same number of atoms of that element on the right side of the equ ...
... lost nor gained in chemical reactions, it states that it simply changes form. For that reason, if you had a certain number of atoms of a certain element on the left side of a chemical reaction equation, then you would have to have the same number of atoms of that element on the right side of the equ ...
Earth Science - Green Local Schools
... Be able to find the number of neutrons, protons, and electrons in an atom Be able to look at a periodic table and gather information about different elements Know how to determine how reactive an element is based on its electrons Chapter 5 – Structure of Matter Chemical bond Making a compound ...
... Be able to find the number of neutrons, protons, and electrons in an atom Be able to look at a periodic table and gather information about different elements Know how to determine how reactive an element is based on its electrons Chapter 5 – Structure of Matter Chemical bond Making a compound ...
Pre Ch15 HW
... For KEY TERMS: Make sure you can define it and/or give an example of it. Pick TWO terms of your choice and actually write the definition or an example. For KEY EQUATIONS AND RELATIONSHIP: Next to EACH, define each term. Be very specific. CHAPTER REVIEW GUIDE Learning Objectives Relevant section (§) ...
... For KEY TERMS: Make sure you can define it and/or give an example of it. Pick TWO terms of your choice and actually write the definition or an example. For KEY EQUATIONS AND RELATIONSHIP: Next to EACH, define each term. Be very specific. CHAPTER REVIEW GUIDE Learning Objectives Relevant section (§) ...
III. ORGANIC CHEMISTRY Reactions
... substitution reactions occur one step at a time; therefore, two substitutions cannot (and do not) take place at the same time (ex. bromine is diatomic, meaning two atoms of bromine are available for a substitution; however, each bromine gets added to a separate hydrocarbon, yielding a HBr molecule i ...
... substitution reactions occur one step at a time; therefore, two substitutions cannot (and do not) take place at the same time (ex. bromine is diatomic, meaning two atoms of bromine are available for a substitution; however, each bromine gets added to a separate hydrocarbon, yielding a HBr molecule i ...
Types of Chemical Reactions
... Types of Chemical Reactions There are many types of chemical reactions. Five of the most common are: synthesis: two or more reactants combine to form a single product. A+BC decomposition: one reactant disintegrates (decomposes) to form two or more products: AB+C single replacement (sometimes calle ...
... Types of Chemical Reactions There are many types of chemical reactions. Five of the most common are: synthesis: two or more reactants combine to form a single product. A+BC decomposition: one reactant disintegrates (decomposes) to form two or more products: AB+C single replacement (sometimes calle ...
Ch. 3 - Chemical Reactions
... One atom of solid zinc reacts with two molecules of aqueous hydrochloric acid to produce one unit of aqueous zinc chloride and one C. Johannesson gas. molecule of hydrogen ...
... One atom of solid zinc reacts with two molecules of aqueous hydrochloric acid to produce one unit of aqueous zinc chloride and one C. Johannesson gas. molecule of hydrogen ...