Organic Reactions 2.1- 2.3 - mccormack-sch4u-2013
... 4) OXIDATION & 5) REDUCTION REACTIONS • Change in the number of H or O atoms bonded to C • Always occur together • One reactant is oxidized while the other is reduced • For now, lets focus on reactant only… ...
... 4) OXIDATION & 5) REDUCTION REACTIONS • Change in the number of H or O atoms bonded to C • Always occur together • One reactant is oxidized while the other is reduced • For now, lets focus on reactant only… ...
Science 9 Unit 2
... the reaction. E.g. a sugar cube takes longer to dissolve than regular refined sugar Energy – the type of energy used will determine how fast the reaction occurs. E.g. if you use electrical energy from a battery the reaction will be faster ...
... the reaction. E.g. a sugar cube takes longer to dissolve than regular refined sugar Energy – the type of energy used will determine how fast the reaction occurs. E.g. if you use electrical energy from a battery the reaction will be faster ...
Exam 2-f06 - Clayton State University
... 8.) The equilibrium constant, Kc for the following gas phase reaction is 0.50 at 600°C. A mixture of HCHO, H and CO is introduced into a flask at 600°C. After a short time, analysis of a small amount of the reaction mixture shows the concentration to be [HCHO] = 1.5M, [H2] = 1.2 M and [CO] = 1.0M. W ...
... 8.) The equilibrium constant, Kc for the following gas phase reaction is 0.50 at 600°C. A mixture of HCHO, H and CO is introduced into a flask at 600°C. After a short time, analysis of a small amount of the reaction mixture shows the concentration to be [HCHO] = 1.5M, [H2] = 1.2 M and [CO] = 1.0M. W ...
Entropy and reaction spontaneity Gibbs free energy
... ∆G = ∆G 0 + RT ln Q For reaction: aA + bB + ... = mM + nN + ... reaction quotient Q is defined as: ...
... ∆G = ∆G 0 + RT ln Q For reaction: aA + bB + ... = mM + nN + ... reaction quotient Q is defined as: ...
lect7
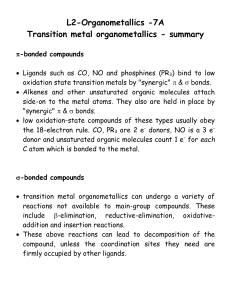
... the 18-electron rule. CO, PR3 are 2 e- donors, NO is a 3 edonor and unsaturated organic molecules count 1 e- for each C atom which is bonded to the metal. ...
... the 18-electron rule. CO, PR3 are 2 e- donors, NO is a 3 edonor and unsaturated organic molecules count 1 e- for each C atom which is bonded to the metal. ...
2. Covalent network
... Dalton’s Law of Partial Pressures: Ptotal=ntotal(RT/V) Used to find pressure of a gas that is in a mixture of gases in a container. Only total number of moles matters. Mole Fraction: X1=P1/Ptotal (Pressure is directly related to moles) ...
... Dalton’s Law of Partial Pressures: Ptotal=ntotal(RT/V) Used to find pressure of a gas that is in a mixture of gases in a container. Only total number of moles matters. Mole Fraction: X1=P1/Ptotal (Pressure is directly related to moles) ...
Oxidation and Reduction - UCLA Chemistry and Biochemistry
... bonds between a carbon and atoms that are less electronegative than carbon (often hydrogen). ...
... bonds between a carbon and atoms that are less electronegative than carbon (often hydrogen). ...
Lecture6-Organometallic Chemistry
... • A catalyzed reaction is faster (or in some cases more specific) than an uncatalyzed version of the same reaction because the catalyst provide a different reaction pathway with a lower activation energy Catalyst efficiency • Turnover frequency : Commonly called the turnover number, N, and define ...
... • A catalyzed reaction is faster (or in some cases more specific) than an uncatalyzed version of the same reaction because the catalyst provide a different reaction pathway with a lower activation energy Catalyst efficiency • Turnover frequency : Commonly called the turnover number, N, and define ...
CH 3 Br + Nu
... B) it involves the formation of the carbocation from elimination of a good leaving group C) a common competing reaction is rearrangement of a less stable carbocation to a more stable carbocation D) the loss of a proton by the carbocation is a fast step E) all of the above ...
... B) it involves the formation of the carbocation from elimination of a good leaving group C) a common competing reaction is rearrangement of a less stable carbocation to a more stable carbocation D) the loss of a proton by the carbocation is a fast step E) all of the above ...
doc
... The difference in the delta G’s controls the product ratio. It is a little harder – and less likely – to form the higher energy TS. Probably the TS resembles the product enough that the TS of the more stable product is also the more stable TS. This is not always the case: Sometimes a less stable pro ...
... The difference in the delta G’s controls the product ratio. It is a little harder – and less likely – to form the higher energy TS. Probably the TS resembles the product enough that the TS of the more stable product is also the more stable TS. This is not always the case: Sometimes a less stable pro ...
EETopic Coversheet Word document
... Know that foam calorimeters are used to measure temperature changes for displacement, dissolving and neutralisation reactions Calculate heat energy for these types of reactions Know that molar enthalpy is used to represent the change in energy of a reaction, per mole of limiting reagent Recall the e ...
... Know that foam calorimeters are used to measure temperature changes for displacement, dissolving and neutralisation reactions Calculate heat energy for these types of reactions Know that molar enthalpy is used to represent the change in energy of a reaction, per mole of limiting reagent Recall the e ...
Chapter 14 Chemical Reactions
... reacted in a closed container, you can show that the mass before and after the reaction is the same. ...
... reacted in a closed container, you can show that the mass before and after the reaction is the same. ...
673 lab three
... A) DISCUSS CHEMICAL REACTIONS: start with a definition and apply the law of conservation of mass in a chemical reaction to the reaction in this lab. Discuss balanced reactions and give three example reactions and SHOW that they are balanced. Clearly indicate the role of COEFFICIENTS., B) DISCUSS CHE ...
... A) DISCUSS CHEMICAL REACTIONS: start with a definition and apply the law of conservation of mass in a chemical reaction to the reaction in this lab. Discuss balanced reactions and give three example reactions and SHOW that they are balanced. Clearly indicate the role of COEFFICIENTS., B) DISCUSS CHE ...
Organic Chemistry
... - All organisms need energy to carry on life processes Energy: the ability to move or change matter (to do work) - Forms: heat, electrical, light, sound, chemical, etc - Energy can be stored or released by chemical reactions (bonds are broken) ...
... - All organisms need energy to carry on life processes Energy: the ability to move or change matter (to do work) - Forms: heat, electrical, light, sound, chemical, etc - Energy can be stored or released by chemical reactions (bonds are broken) ...