Writing and Balancing Chemical Equations
... We will learn: a) the 5 major types. We will be able to: b) predict the products. For some, we will be able to: c) predict whether or not they will happen at all. ...
... We will learn: a) the 5 major types. We will be able to: b) predict the products. For some, we will be able to: c) predict whether or not they will happen at all. ...
1 Chemical Reactions: Chemistry Word Equations • Write the names
... – O= O= Balancing Rules 1. Determine the correct ____________________ for all the reactants and products. 2. Write the _______________________ equation. (Reactants on left, products on right, yield sign in between. If two or more reactants/products are involved, separate their formulas with plus sig ...
... – O= O= Balancing Rules 1. Determine the correct ____________________ for all the reactants and products. 2. Write the _______________________ equation. (Reactants on left, products on right, yield sign in between. If two or more reactants/products are involved, separate their formulas with plus sig ...
doc CHEM 222 Lab exam with Answers
... temperature and then allowing them to come back out of solution. 2.__T___ The purpose of refluxing is to carry out a reaction at the boiling point of the solvent. 3.__F___ All chemical reactions must take place in solution. 4.__T___ When a carbene is formed in the presence of an alkene, a cyclopropa ...
... temperature and then allowing them to come back out of solution. 2.__T___ The purpose of refluxing is to carry out a reaction at the boiling point of the solvent. 3.__F___ All chemical reactions must take place in solution. 4.__T___ When a carbene is formed in the presence of an alkene, a cyclopropa ...
Ch. 10 – Stoichiometry Stoichiometry – relates molar ratios between
... Ch. 10 – Stoichiometry Stoichiometry – relates molar ratios between reactants and products in a chemical equation ...
... Ch. 10 – Stoichiometry Stoichiometry – relates molar ratios between reactants and products in a chemical equation ...
DESIGN OF CHIRAL IMINO- AND AMINOPYRIDINE LIGANDS
... On the other hand, reactions allowing the formation of C-C bonds are of great importance in organic synthesis because they allow to increase the structural complexity of the molecules. Among such reactions, the Henry or nitroaldol reaction 3 constitutes one of the most useful methodologies for the f ...
... On the other hand, reactions allowing the formation of C-C bonds are of great importance in organic synthesis because they allow to increase the structural complexity of the molecules. Among such reactions, the Henry or nitroaldol reaction 3 constitutes one of the most useful methodologies for the f ...
Organic Chemistry (HL) Revision Questions
... Both propan-1-ol and propan-2-ol can be oxidized in aqueous solution by potassium dichromate(VI). State any necessary conditions for the oxidation to occur and describe the colour change during the oxidation process. ...
... Both propan-1-ol and propan-2-ol can be oxidized in aqueous solution by potassium dichromate(VI). State any necessary conditions for the oxidation to occur and describe the colour change during the oxidation process. ...
KEY Final Exam Review - Iowa State University
... a. What is the rate law for the reaction? k[BF3][NH3] seen by exp 1&2;4&5 b. What is the overall order of the reaction? 2 c. Calculate the Rate constant with proper units. Using exp 1 k=(0.2130)M/s/(0.250M)(0.250M)=3.41M-1s-1 could use any of the five to calculate this. kave=3.408M-1s-1 d. What is t ...
... a. What is the rate law for the reaction? k[BF3][NH3] seen by exp 1&2;4&5 b. What is the overall order of the reaction? 2 c. Calculate the Rate constant with proper units. Using exp 1 k=(0.2130)M/s/(0.250M)(0.250M)=3.41M-1s-1 could use any of the five to calculate this. kave=3.408M-1s-1 d. What is t ...
A-level Paper 2 Practice Paper 1 - A
... A reaction mechanism is a series of steps by which an overall reaction may proceed. The reactions occurring in these steps may be deduced from a study of reaction rates. Experimental evidence about initial rates leads to a rate equation. A mechanism is then proposed which agrees with this rate equat ...
... A reaction mechanism is a series of steps by which an overall reaction may proceed. The reactions occurring in these steps may be deduced from a study of reaction rates. Experimental evidence about initial rates leads to a rate equation. A mechanism is then proposed which agrees with this rate equat ...
File - LSAmockscience
... • When one element replaces another element in a compound A + BC AC + B Element + compound new element + new compound ...
... • When one element replaces another element in a compound A + BC AC + B Element + compound new element + new compound ...
File - IGCSE STUDY BANK
... I was once asked "what is the opposite of a catalyst?" There is no real opposite to a catalyst, other than the uncatalysed reaction! The word catalyst means changing the rate of a reaction with some other material 'added to' or in 'contact with' the reaction mixture. There are the two phrases you ma ...
... I was once asked "what is the opposite of a catalyst?" There is no real opposite to a catalyst, other than the uncatalysed reaction! The word catalyst means changing the rate of a reaction with some other material 'added to' or in 'contact with' the reaction mixture. There are the two phrases you ma ...
Chemical Synthesis
... • Should: Be able to make a soluble salt. • Could: Explain all the stages and techniques used. • Keywords: Synthesis, techniques, plan reactant and product. ...
... • Should: Be able to make a soluble salt. • Could: Explain all the stages and techniques used. • Keywords: Synthesis, techniques, plan reactant and product. ...
PDF(343KB)
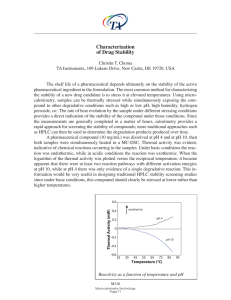
... A pharmaceutical compound (10 mg/mL) was dissolved at pH 4 and at pH 10, then both samples were simultaneously heated in a MC-DSC. Thermal activity was evident, indicative of chemical reactions occurring in the samples. Under basic conditions the reaction was endothermic, while in acidic conditions ...
... A pharmaceutical compound (10 mg/mL) was dissolved at pH 4 and at pH 10, then both samples were simultaneously heated in a MC-DSC. Thermal activity was evident, indicative of chemical reactions occurring in the samples. Under basic conditions the reaction was endothermic, while in acidic conditions ...
Enzymes
... The active site places substrates in the correct orientation for the reaction. As the active site binds the substrate, it may put stress on bonds that must be broken, making it easier to reach the transition state. R groups at the active site may create a conducive ...
... The active site places substrates in the correct orientation for the reaction. As the active site binds the substrate, it may put stress on bonds that must be broken, making it easier to reach the transition state. R groups at the active site may create a conducive ...