Nuclear/Heat
... differences in density. c. the force of buoyancy. d. electromagnetic radiation. 9. The type of heat transfer which requires no matter in order for it to occur is called: a. insulation. Give an example: b. conduction. c. convection. d. radiation. 10. The type of heat transfer called convection happen ...
... differences in density. c. the force of buoyancy. d. electromagnetic radiation. 9. The type of heat transfer which requires no matter in order for it to occur is called: a. insulation. Give an example: b. conduction. c. convection. d. radiation. 10. The type of heat transfer called convection happen ...
People Search for Review
... 4. What is an isotope? What makes an isotope of one element different from a different isotope of the same element? ...
... 4. What is an isotope? What makes an isotope of one element different from a different isotope of the same element? ...
Exam #2
... Each of the following statements describes the significance of a certain key experiment or idea. Which one is not correct? (a) (b) (c) (d) (e) ...
... Each of the following statements describes the significance of a certain key experiment or idea. Which one is not correct? (a) (b) (c) (d) (e) ...
Study Guide Answers
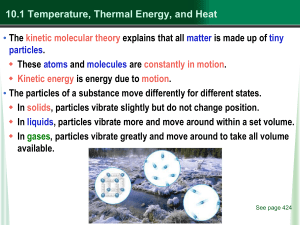
... 4. Your bare feet cool on a cement floor due to _c___ a. Convection b. Radiation c. Conduction 5. __Radiation__________ is the transfer of energy through space. 6. _Convection___________ is the transfer of energy by the movement of a liquid or a gas from one place to another. 7. The total kinetic en ...
... 4. Your bare feet cool on a cement floor due to _c___ a. Convection b. Radiation c. Conduction 5. __Radiation__________ is the transfer of energy through space. 6. _Convection___________ is the transfer of energy by the movement of a liquid or a gas from one place to another. 7. The total kinetic en ...
Lecture 2 Applications of the free electron gas model
... metals, such as Al, have a positive RH value. Therefore it would appear that in some materials electrons are positively charged, contrary to what we expect. In order to resolve this difficulty we need to develop a better theory of electrons in metals. This will be our goal for the next few lectures. ...
... metals, such as Al, have a positive RH value. Therefore it would appear that in some materials electrons are positively charged, contrary to what we expect. In order to resolve this difficulty we need to develop a better theory of electrons in metals. This will be our goal for the next few lectures. ...
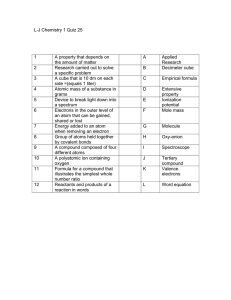
Chemistry I Unit Review: The Atom Text Chapters 2 and 7 1. The
... How many protons are in the nucleus of each of the following elements? (also known as the atomic number) a. uranium c. helium b. selenium d. bohrium Give the number of neutrons in each of the following isotopes. (what is an isotope?) a. titanium-46 c. 3416S b. nitrogen-15 d. 6529Cu Fill in the blank ...
... How many protons are in the nucleus of each of the following elements? (also known as the atomic number) a. uranium c. helium b. selenium d. bohrium Give the number of neutrons in each of the following isotopes. (what is an isotope?) a. titanium-46 c. 3416S b. nitrogen-15 d. 6529Cu Fill in the blank ...
1. Millikan did his experiments with the balance of
... 6. a.) The condensed ground-state electron configuration of Mo3+ : [Kr] 5s2, 4d1 Mo3+ is paramagnetic because in the d orbital there are unpaired electrons. b.) The condensed ground-state electron configuration of Au+ : [Xe] 6s2, 4f14,5d8 Au+ is paramagnetic because in the d orbital there are unpai ...
... 6. a.) The condensed ground-state electron configuration of Mo3+ : [Kr] 5s2, 4d1 Mo3+ is paramagnetic because in the d orbital there are unpaired electrons. b.) The condensed ground-state electron configuration of Au+ : [Xe] 6s2, 4f14,5d8 Au+ is paramagnetic because in the d orbital there are unpai ...
Chemistry CP Final Exam Review #2
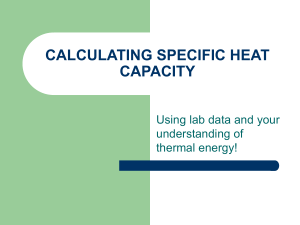
... Chemistry CP Final Exam Review #2 Chapter 10: Energy Define the following terms: energy, potential energy, kinetic energy, radiant energy, Law of conservation of energy, state function, temperature, heat, exothermic reaction, endothermic reaction, calorie, specific heat, enthalpy, calorimeter, Hess’ ...
... Chemistry CP Final Exam Review #2 Chapter 10: Energy Define the following terms: energy, potential energy, kinetic energy, radiant energy, Law of conservation of energy, state function, temperature, heat, exothermic reaction, endothermic reaction, calorie, specific heat, enthalpy, calorimeter, Hess’ ...
Homework 2
... 4. Compton effect An incident photon with wave vector k0 is scattered from a particle with rest mass m0 and initial momentum p0 = 0. The scattered photon has the wave vector k. Based on the conservation of the relativistic energy and momentum, determine the shift λ − λ0 of the wave length of the sca ...
... 4. Compton effect An incident photon with wave vector k0 is scattered from a particle with rest mass m0 and initial momentum p0 = 0. The scattered photon has the wave vector k. Based on the conservation of the relativistic energy and momentum, determine the shift λ − λ0 of the wave length of the sca ...
Primary electrons make random elastic and inelastic collision either
... Inelastic interactions (scattering), result in the transfer of energy from primary electrons to the atoms of the sample, and this energy exchange is limited to atomic electrons, rather than nuclei… Inelastic scattering decreases the kinetic energy of the bombarding electron; meanwhile the deviation ...
... Inelastic interactions (scattering), result in the transfer of energy from primary electrons to the atoms of the sample, and this energy exchange is limited to atomic electrons, rather than nuclei… Inelastic scattering decreases the kinetic energy of the bombarding electron; meanwhile the deviation ...
Heat transfer physics

Heat transfer physics describes the kinetics of energy storage, transport, and transformation by principal energy carriers: phonons (lattice vibration waves), electrons, fluid particles, and photons. Heat is energy stored in temperature-dependent motion of particles including electrons, atomic nuclei, individual atoms, and molecules. Heat is transferred to and from matter by the principal energy carriers. The state of energy stored within matter, or transported by the carriers, is described by a combination of classical and quantum statistical mechanics. The energy is also transformed (converted) among various carriers.The heat transfer processes (or kinetics) are governed by the rates at which various related physical phenomena occur, such as (for example) the rate of particle collisions in classical mechanics. These various states and kinetics determine the heat transfer, i.e., the net rate of energy storage or transport. Governing these process from the atomic level (atom or molecule length scale) to macroscale are the laws of thermodynamics, including conservation of energy.