PHYS4330 Theoretical Mechanics HW #1 Due 6 Sept 2011
... and numerically determine the period as a function of the (dimensionless) variable ym ≡ xm /a. It is easiest to write the period T as a definite integral over one quarter of the period, and then multiply by four. Your computer can do the integral numerically. Make a plot of T versus ym and show that ...
... and numerically determine the period as a function of the (dimensionless) variable ym ≡ xm /a. It is easiest to write the period T as a definite integral over one quarter of the period, and then multiply by four. Your computer can do the integral numerically. Make a plot of T versus ym and show that ...
Chemistry 2: matter is made up of atoms
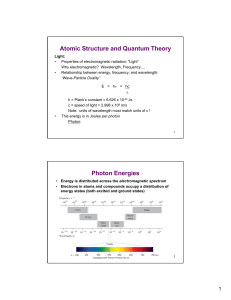
... spectrum, from radio waves, AM, FM, microwaves, infrared, visible spectrum, UV, X-rays, to gamma rays, which are very energetic and have very short wave-lengths ...
... spectrum, from radio waves, AM, FM, microwaves, infrared, visible spectrum, UV, X-rays, to gamma rays, which are very energetic and have very short wave-lengths ...
- Lexington JHS
... Compounds – a pure substance made of two or more elements chemically combined in a set ratio. (different properties than single elements) H2O, CO2,C6H12O22 Chemical formula-shows ratio (shorthand) ...
... Compounds – a pure substance made of two or more elements chemically combined in a set ratio. (different properties than single elements) H2O, CO2,C6H12O22 Chemical formula-shows ratio (shorthand) ...
Chem 1151
... concentration of As(III) in an unknown sample. What is the molarity of As(III) if 33.45 mL of 0.125M KBrO3 is needed to titrate 50.0 mL of the As(III) solution? The balanced chemical equation is H3AsO3(aq) + BrO3-(aq) Br -(aq) + 3H3AsO4(aq) ...
... concentration of As(III) in an unknown sample. What is the molarity of As(III) if 33.45 mL of 0.125M KBrO3 is needed to titrate 50.0 mL of the As(III) solution? The balanced chemical equation is H3AsO3(aq) + BrO3-(aq) Br -(aq) + 3H3AsO4(aq) ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... b. If the bond length of C-C is 1.48 Ǻ and C=C is 1.34 Ǻ, calculate the penetration effect. ...
... b. If the bond length of C-C is 1.48 Ǻ and C=C is 1.34 Ǻ, calculate the penetration effect. ...
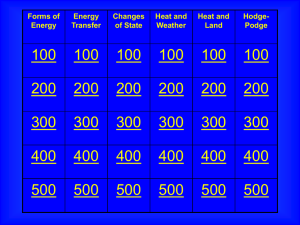
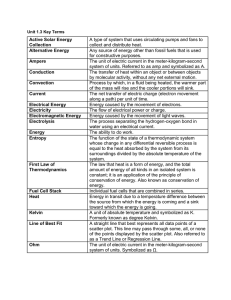
Unit 1.3 Key Terms Active Solar Energy Collection A type of system
... system of units. Referred to as amp and symbolized as A. The transfer of heat within an object or between objects by molecular activity, without any net external motion. Process by which, in a fluid being heated, the warmer part of the mass will rise and the cooler portions will sink. The net transf ...
... system of units. Referred to as amp and symbolized as A. The transfer of heat within an object or between objects by molecular activity, without any net external motion. Process by which, in a fluid being heated, the warmer part of the mass will rise and the cooler portions will sink. The net transf ...
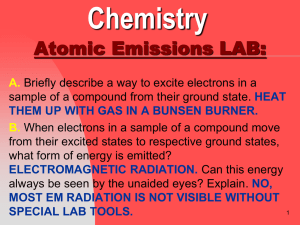
Atomic Emissions LAB Questions
... Atomic Emissions LAB: G. How would the width (spacing) between energy levels in the electron cloud for different elements affect the amount of energy released as electrons move from their excited states to respective ground states? EACH ELEMENT HAS A UNIQUE SPACING OF ENERGY LEVELS IN ITS ELECTRON C ...
... Atomic Emissions LAB: G. How would the width (spacing) between energy levels in the electron cloud for different elements affect the amount of energy released as electrons move from their excited states to respective ground states? EACH ELEMENT HAS A UNIQUE SPACING OF ENERGY LEVELS IN ITS ELECTRON C ...
Unit 1 Inorganic Flashcards
... A technique for determining the concentration of a particular metal element in a sample. The electrons are promoted to higher energy levels by absorbing energy, and the wavelength of the absorbed energy can be used to determine which element is present. The intensity of the absorbed light can be use ...
... A technique for determining the concentration of a particular metal element in a sample. The electrons are promoted to higher energy levels by absorbing energy, and the wavelength of the absorbed energy can be used to determine which element is present. The intensity of the absorbed light can be use ...
Concepts for specific heat
... distributed between the kinetic and potential energy. (This only holds for quadratic potentials!) In thermal equilibrium each oscillation mode has the internal energy E = kB T ...
... distributed between the kinetic and potential energy. (This only holds for quadratic potentials!) In thermal equilibrium each oscillation mode has the internal energy E = kB T ...
Nearly Free Electron Approximation
... This is largely brought on by the lattice of positively charged nuclei that can interact with the electrons, so essentially the electrons are not free ie (nearly free approximation), but periodically disrupted by an attractive potential. The wavefunction for an electron in a periodic potential is gi ...
... This is largely brought on by the lattice of positively charged nuclei that can interact with the electrons, so essentially the electrons are not free ie (nearly free approximation), but periodically disrupted by an attractive potential. The wavefunction for an electron in a periodic potential is gi ...
9.1 Heat and Temperature
... B. Temperature can be measured in Fahrenheit, Celsius, or in Kelvin. 1. K = 273 + OC 2. OC = (OF -32) x 5/9 3. OF = (9/5 x OC) +32 IV. Specific Heat (CP) A value of energy associated with that specific substance. A. The amount of energy required to raise the temperature of a one gram sample of a sub ...
... B. Temperature can be measured in Fahrenheit, Celsius, or in Kelvin. 1. K = 273 + OC 2. OC = (OF -32) x 5/9 3. OF = (9/5 x OC) +32 IV. Specific Heat (CP) A value of energy associated with that specific substance. A. The amount of energy required to raise the temperature of a one gram sample of a sub ...
AP Chemistry Study Guide – Chapter 7, Atomic Structure
... 6) Account for each of the following in terms of principles of atomic structure, including the number, properties, and arrangements of subatomic particles. (a) The second ionization energy of sodium is about three times greater than the second ionization energy of magnesium. (b) The difference betwe ...
... 6) Account for each of the following in terms of principles of atomic structure, including the number, properties, and arrangements of subatomic particles. (a) The second ionization energy of sodium is about three times greater than the second ionization energy of magnesium. (b) The difference betwe ...
Thermodynamics lesson 1 Tempersture
... • oF is for old people, like pounds and ounces BUT conversion is a skill so lets not dispose of it all together • R just for some US engineers • oC not C. Centigrade just means that, we want Celsius, and degress at that. • K is not oK as it is absolute. small point but important ...
... • oF is for old people, like pounds and ounces BUT conversion is a skill so lets not dispose of it all together • R just for some US engineers • oC not C. Centigrade just means that, we want Celsius, and degress at that. • K is not oK as it is absolute. small point but important ...
Ist law of thermodynamics
... water or an iceberg? And which system has a larger internal energy? ...
... water or an iceberg? And which system has a larger internal energy? ...
Heat transfer physics

Heat transfer physics describes the kinetics of energy storage, transport, and transformation by principal energy carriers: phonons (lattice vibration waves), electrons, fluid particles, and photons. Heat is energy stored in temperature-dependent motion of particles including electrons, atomic nuclei, individual atoms, and molecules. Heat is transferred to and from matter by the principal energy carriers. The state of energy stored within matter, or transported by the carriers, is described by a combination of classical and quantum statistical mechanics. The energy is also transformed (converted) among various carriers.The heat transfer processes (or kinetics) are governed by the rates at which various related physical phenomena occur, such as (for example) the rate of particle collisions in classical mechanics. These various states and kinetics determine the heat transfer, i.e., the net rate of energy storage or transport. Governing these process from the atomic level (atom or molecule length scale) to macroscale are the laws of thermodynamics, including conservation of energy.