Artificial Radioactivity Artificial Radioactivity And Q
... exert large coulomb forces on the atomic electrons and impart energy to them. The energy transfer may be sufficient to allow the electron to leave the parent atom, and so cause ionization, which is completed within about 10-15 s. Alternatively, the atomic electron may be excited to a higher state. T ...
... exert large coulomb forces on the atomic electrons and impart energy to them. The energy transfer may be sufficient to allow the electron to leave the parent atom, and so cause ionization, which is completed within about 10-15 s. Alternatively, the atomic electron may be excited to a higher state. T ...
Work sheet –chapter 2 CLASS - XI CHEMISTRY (Structure of Atom
... 5. What did Einstein explain about photoelectric effect? 6. What is the relation between kinetic energy and frequency of the photoelectrons? 7. Calculate energy of 2mole of photons of radiation whose frequency is 51014Hz. 8. What is emission and absorption spectra? 9. What transition in the hydroge ...
... 5. What did Einstein explain about photoelectric effect? 6. What is the relation between kinetic energy and frequency of the photoelectrons? 7. Calculate energy of 2mole of photons of radiation whose frequency is 51014Hz. 8. What is emission and absorption spectra? 9. What transition in the hydroge ...
Topic 2: Molecular Dynamics of Lennard
... Newton’s equations of motion for the system are integrated numerically. If the system is in equilibirium, static properties such as temperature and pressure are measured as averages over time. Dynamical properties such as heat transport, or relaxation of systems far from equilibrium, can also be stu ...
... Newton’s equations of motion for the system are integrated numerically. If the system is in equilibirium, static properties such as temperature and pressure are measured as averages over time. Dynamical properties such as heat transport, or relaxation of systems far from equilibrium, can also be stu ...
Mass Balance for Open System
... The rate of change of energy within the control volume equals the net rate of energy transfer into the control volume. Stream flowing in and out of the control volume are associated with energy. Each stream will have total energy of; ...
... The rate of change of energy within the control volume equals the net rate of energy transfer into the control volume. Stream flowing in and out of the control volume are associated with energy. Each stream will have total energy of; ...
HEAT- Chapter 9
... temperature decreases; the exception is water Solids tend to have the smallest coefficient of volume Coefficient of Volume Expansion- a number assigned to different material to show the thermal expansion characteristic of the material Gases have highest coefficient; solids the lowest ...
... temperature decreases; the exception is water Solids tend to have the smallest coefficient of volume Coefficient of Volume Expansion- a number assigned to different material to show the thermal expansion characteristic of the material Gases have highest coefficient; solids the lowest ...
Basics of Semiconductors_1
... constructing an analogy to the behavior of a free particle with that mass It is represented as m* and for electron in energy E and wave vector k is given ...
... constructing an analogy to the behavior of a free particle with that mass It is represented as m* and for electron in energy E and wave vector k is given ...
Lecture_3 - Department of Mathematics
... Heat Q is thermal energy transferred to a system from its environment, Q > 0, Q < 0 when the system temperature is lower, higher than environment’s, it can be associated with a change of temperature ...
... Heat Q is thermal energy transferred to a system from its environment, Q > 0, Q < 0 when the system temperature is lower, higher than environment’s, it can be associated with a change of temperature ...
Bohr`s Model and the Balmer Equation
... This classical treatment y shows us that an electron’s energy is a function of the distance, r, between the electron and the nucleus; however, there is nothing in this treatment that limits the radius of an electron’s orbit or its energy. Bohr quantized the atom by assuming that an electron’s ...
... This classical treatment y shows us that an electron’s energy is a function of the distance, r, between the electron and the nucleus; however, there is nothing in this treatment that limits the radius of an electron’s orbit or its energy. Bohr quantized the atom by assuming that an electron’s ...
Atomic Theory (Or a quick Chemistry Review)
... Atomic Theory Q: What does science study? A: The natural world, the physical universe Q: What are the components of the P.U? A: matter, energy, forces ...
... Atomic Theory Q: What does science study? A: The natural world, the physical universe Q: What are the components of the P.U? A: matter, energy, forces ...
Chem 1st Sem Rev Ch2
... c. father of the modern atomic theory, everything made of atoms d. planetary model of the atom, electrons move around the nucleus like planets around sun. e. plum pudding model of the atom: atom looks like chocolate chip cookie f. gold foil experiment – atoms have a dense core called nucleus g. he g ...
... c. father of the modern atomic theory, everything made of atoms d. planetary model of the atom, electrons move around the nucleus like planets around sun. e. plum pudding model of the atom: atom looks like chocolate chip cookie f. gold foil experiment – atoms have a dense core called nucleus g. he g ...
Ch 16 Thermal Energy and Heat
... • In the 1700’s scientists thought heat was a fluid called a caloric that flowed between objects. • In 1798, the scientist Count Rumford concluded, from his observations, that heat could not be a kind of matter but instead was related to the motion of objects ...
... • In the 1700’s scientists thought heat was a fluid called a caloric that flowed between objects. • In 1798, the scientist Count Rumford concluded, from his observations, that heat could not be a kind of matter but instead was related to the motion of objects ...
Starter
... ground the greater the kinetic energy. • KE=1/2(m)v2 KE= kinetic m= mass v= velocity Unit=Joule ...
... ground the greater the kinetic energy. • KE=1/2(m)v2 KE= kinetic m= mass v= velocity Unit=Joule ...
Verdana 30 pt
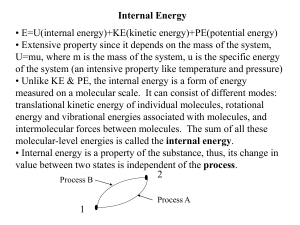
... behavior with relatively simple and accurate laws, based on measures of volume, pressure and temperature, said state quantities; these, we add the internal energy U of an ideal gas, which is all kinetic and depends only on the temperature. ...
... behavior with relatively simple and accurate laws, based on measures of volume, pressure and temperature, said state quantities; these, we add the internal energy U of an ideal gas, which is all kinetic and depends only on the temperature. ...
Verdana 30 pt - Liceo Statale Aprosio
... behavior with relatively simple and accurate laws, based on measures of volume, pressure and temperature, said state quantities; these, we add the internal energy U of an ideal gas, which is all kinetic and depends only on the temperature. ...
... behavior with relatively simple and accurate laws, based on measures of volume, pressure and temperature, said state quantities; these, we add the internal energy U of an ideal gas, which is all kinetic and depends only on the temperature. ...
Electrons_Holes
... point on the valence band. The difference between these two points is still the bandgap energy, Eg. If the free electron drops down from the lowest point in the conduction band in the highest point in the valence band, energy must be released using some particle that has momentum. The particle that ...
... point on the valence band. The difference between these two points is still the bandgap energy, Eg. If the free electron drops down from the lowest point in the conduction band in the highest point in the valence band, energy must be released using some particle that has momentum. The particle that ...
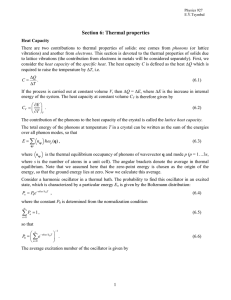
6. Thermal Properties
... CV on temperature has already been studied in detail, while the velocity v is found to be essentially insensitive to temperature. The mean free path l depends strongly on temperature. Indeed, l is the average distance the phonon travels between two successive collisions. Three important mechanisms m ...
... CV on temperature has already been studied in detail, while the velocity v is found to be essentially insensitive to temperature. The mean free path l depends strongly on temperature. Indeed, l is the average distance the phonon travels between two successive collisions. Three important mechanisms m ...
Thermochemistry
... of water by one Celsius degree. (Food you eat is measured in Kilocalories which is abbreviated C). • Joule (J)-the SI unit of energy • 1 c=4.184J ...
... of water by one Celsius degree. (Food you eat is measured in Kilocalories which is abbreviated C). • Joule (J)-the SI unit of energy • 1 c=4.184J ...
(8%) Write (a) the mass-balance expression and (b) the charge-balance equation
... a. Express the normalization factor N for the molecular orbital y = N (XA+~XB) in terms of the-parameterh and the overlap integral S between the two atomic orbitals, X A and XB. N = b. .Writedown the spin part of the wavefunction 4, for the vJence-bond wavefunction for Hz in a excited state with Sz= ...
... a. Express the normalization factor N for the molecular orbital y = N (XA+~XB) in terms of the-parameterh and the overlap integral S between the two atomic orbitals, X A and XB. N = b. .Writedown the spin part of the wavefunction 4, for the vJence-bond wavefunction for Hz in a excited state with Sz= ...
Heat transfer physics

Heat transfer physics describes the kinetics of energy storage, transport, and transformation by principal energy carriers: phonons (lattice vibration waves), electrons, fluid particles, and photons. Heat is energy stored in temperature-dependent motion of particles including electrons, atomic nuclei, individual atoms, and molecules. Heat is transferred to and from matter by the principal energy carriers. The state of energy stored within matter, or transported by the carriers, is described by a combination of classical and quantum statistical mechanics. The energy is also transformed (converted) among various carriers.The heat transfer processes (or kinetics) are governed by the rates at which various related physical phenomena occur, such as (for example) the rate of particle collisions in classical mechanics. These various states and kinetics determine the heat transfer, i.e., the net rate of energy storage or transport. Governing these process from the atomic level (atom or molecule length scale) to macroscale are the laws of thermodynamics, including conservation of energy.