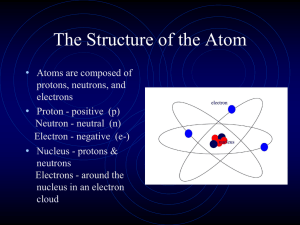
Atomic Structure
... Earth - cool, heavy Water - wet Blend these in different proportions to get all substances ...
... Earth - cool, heavy Water - wet Blend these in different proportions to get all substances ...
Periodic Table Notes
... 2. All matter is made of atoms, and matter is all around you. Matter comes in 4 states: (1) Solid, (2) Liquid, (3) Gas, and (4) Plasma. If an item is not one of these four states, then it is not matter, and it is therefore not made up of atoms. Select the item below that IS NOT made of atoms. a.Plan ...
... 2. All matter is made of atoms, and matter is all around you. Matter comes in 4 states: (1) Solid, (2) Liquid, (3) Gas, and (4) Plasma. If an item is not one of these four states, then it is not matter, and it is therefore not made up of atoms. Select the item below that IS NOT made of atoms. a.Plan ...
c1l2ch06
... The periodic law: When elements are arranged in order of increasing atomic number, there is a periodic repetition of their physical and chemical properties. ...
... The periodic law: When elements are arranged in order of increasing atomic number, there is a periodic repetition of their physical and chemical properties. ...
CH 7 Periodic Table Properties
... • valence electrons • Effective nuclear charge (net charge on an electron) Protons-electrons attraction (increases) Electron-electron repulsion (decreases) Penetration of the orbitals (greater penetration – increases the Zeff) ...
... • valence electrons • Effective nuclear charge (net charge on an electron) Protons-electrons attraction (increases) Electron-electron repulsion (decreases) Penetration of the orbitals (greater penetration – increases the Zeff) ...
Atomic Theory
... 2.1.3 Define the terms mass number (A), atomic number (Z) and isotopes of an element 2.1.4 Deduce the symbol for an isotope given its mass number and atomic number 2.1.5 Calculate the number of protons, neutrons and electrons in atoms and ions from the mass number, atomic number and charge. 2.1.6 Co ...
... 2.1.3 Define the terms mass number (A), atomic number (Z) and isotopes of an element 2.1.4 Deduce the symbol for an isotope given its mass number and atomic number 2.1.5 Calculate the number of protons, neutrons and electrons in atoms and ions from the mass number, atomic number and charge. 2.1.6 Co ...
PPTB&W - Gmu - George Mason University
... state of the elements, thus similar chemical properties The letters A and B were designated to the left (A) and right (B) part of the table ...
... state of the elements, thus similar chemical properties The letters A and B were designated to the left (A) and right (B) part of the table ...
filled in teacher version, level 1 only
... (side note- it was actually Geiger- Marsden Experiment. Scientists Hans G. and undergraduate Ernest M. worked for Rutherford.) “It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15inch shell at a piece of tissue paper and it ...
... (side note- it was actually Geiger- Marsden Experiment. Scientists Hans G. and undergraduate Ernest M. worked for Rutherford.) “It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15inch shell at a piece of tissue paper and it ...
THE DISCOVERY OF ATOMIC PARTICLES
... their masses, lighter ions reaching higher speeds. The path of the ions is bent as they travel between the poles of a variable electromagnet. As the magnetic field is varied, each type of positive ion is, in turn, directed to a detector which produces electric signals integrated into a ‘mass spectru ...
... their masses, lighter ions reaching higher speeds. The path of the ions is bent as they travel between the poles of a variable electromagnet. As the magnetic field is varied, each type of positive ion is, in turn, directed to a detector which produces electric signals integrated into a ‘mass spectru ...
chapter 4 presentation
... Magnesium has three isotopes. 78.99% magnesium 24 with a mass of 23.9850 amu, 10.00% magnesium 25 with a mass of 24.9858 amu, and the rest magnesium 25 with a mass of 25.9826 amu. What is the atomic mass of magnesium? If not told otherwise, the mass of the isotope is the mass number in amu ...
... Magnesium has three isotopes. 78.99% magnesium 24 with a mass of 23.9850 amu, 10.00% magnesium 25 with a mass of 24.9858 amu, and the rest magnesium 25 with a mass of 25.9826 amu. What is the atomic mass of magnesium? If not told otherwise, the mass of the isotope is the mass number in amu ...
File - Flipped Out Science with Mrs. Thomas!
... Electrons do not orbit the nucleus of an atom like planets orbit the Sun Scale does not represent the actual size of an atom So, Why do we still learn the Bohr Model? Using this model makes it easier to understand the general structure of ...
... Electrons do not orbit the nucleus of an atom like planets orbit the Sun Scale does not represent the actual size of an atom So, Why do we still learn the Bohr Model? Using this model makes it easier to understand the general structure of ...
atom - eReportz
... The particle theory of matter was supported as early as 400 b.c. by certain Greek thinkers, such as Democritus. He called nature’s basic particle an atom, based on the Greek word meaning “indivisible.” Aristotle was part of the generation that succeeded Democritus. His ideas had a lasting impact on ...
... The particle theory of matter was supported as early as 400 b.c. by certain Greek thinkers, such as Democritus. He called nature’s basic particle an atom, based on the Greek word meaning “indivisible.” Aristotle was part of the generation that succeeded Democritus. His ideas had a lasting impact on ...
Document
... accelerator at nearly the speed of light. By crashing protons into antiprotons or into fixed targets, researchers can create new and different particles to study. Creating a new particle, however, requires an enormous amount of energy. The Tevatron is unique because it can accelerate particles to en ...
... accelerator at nearly the speed of light. By crashing protons into antiprotons or into fixed targets, researchers can create new and different particles to study. Creating a new particle, however, requires an enormous amount of energy. The Tevatron is unique because it can accelerate particles to en ...
Chemical Change
... • Democritus’s ideas did not explain chemical behavior. • Lacked experimental support, because his approach was not based on scientific method. ...
... • Democritus’s ideas did not explain chemical behavior. • Lacked experimental support, because his approach was not based on scientific method. ...
Chemistry SOL Review
... • Electron energy levels are wave functions. • Electrons are found in orbitals, regions of space where an electron is most likely to be found. • You can’t know both where the electron is and where it is going at the same time. • Electrons buzz around the nucleus like gnats buzzing around your head. ...
... • Electron energy levels are wave functions. • Electrons are found in orbitals, regions of space where an electron is most likely to be found. • You can’t know both where the electron is and where it is going at the same time. • Electrons buzz around the nucleus like gnats buzzing around your head. ...
ch14 lecture 7e
... Zeff increases for the larger 3A elements due to poor shielding by d and f electrons. The larger 3A elements have smaller atomic radii and larger ionization energies and electronegativities than expected. These properties influence the physical and chemical behavior of these elements. ...
... Zeff increases for the larger 3A elements due to poor shielding by d and f electrons. The larger 3A elements have smaller atomic radii and larger ionization energies and electronegativities than expected. These properties influence the physical and chemical behavior of these elements. ...
PPT - George Mason University
... chemical properties The letters A and B were designated to the left (A) and right (B) part of the table ...
... chemical properties The letters A and B were designated to the left (A) and right (B) part of the table ...
The Structure of Atoms
... • When two protons are extremely close to each other, there is a strong attraction between them. • A similar attraction exists when neutrons are very close to each other or when protons and neutrons are very close together. • The short-range proton-neutron, proton-proton, and neutron-neutron forces ...
... • When two protons are extremely close to each other, there is a strong attraction between them. • A similar attraction exists when neutrons are very close to each other or when protons and neutrons are very close together. • The short-range proton-neutron, proton-proton, and neutron-neutron forces ...
ISOTOPIC NOTATION isotopes are atoms with the same number of
... a) 53 neutrons b) 53 protons C) 26 neutrons & 27 protons d) 26 protons & 27 neutrons _______2. The mass of one atom of an isotope is 9.746 x 10-23 g. One atomic mass unit has the mass of 1.6606 x 10-24 g. The atomic mass of this isotope is a) 5.870 amu b) 16.18 amu c) 58.69 amu d) 1.627 amu ...
... a) 53 neutrons b) 53 protons C) 26 neutrons & 27 protons d) 26 protons & 27 neutrons _______2. The mass of one atom of an isotope is 9.746 x 10-23 g. One atomic mass unit has the mass of 1.6606 x 10-24 g. The atomic mass of this isotope is a) 5.870 amu b) 16.18 amu c) 58.69 amu d) 1.627 amu ...
atoms - K12Share
... seen may look like the one below. It shows the parts and structure of the atom. Even though we do not know what an atom looks like, scientific models must be based on evidence. ...
... seen may look like the one below. It shows the parts and structure of the atom. Even though we do not know what an atom looks like, scientific models must be based on evidence. ...