Name
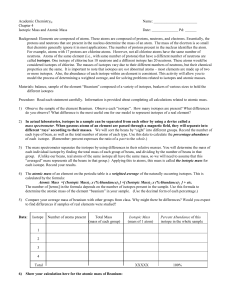
... • Isotopes are atoms of the same element that have different masses. • The isotopes of a particular element all have the same number of protons and electrons but different numbers of neutrons. • Most of the elements consist of mixtures of isotopes. Mass Number • The mass number is the total number o ...
... • Isotopes are atoms of the same element that have different masses. • The isotopes of a particular element all have the same number of protons and electrons but different numbers of neutrons. • Most of the elements consist of mixtures of isotopes. Mass Number • The mass number is the total number o ...
Chapter 7 The Development of the Periodic Table
... based on similar chemical properties. – The term period suggests that the elements show regular patterns in their chemical properties ...
... based on similar chemical properties. – The term period suggests that the elements show regular patterns in their chemical properties ...
Jeopardy - SchoolRack
... $400 Answer from Model of the Atom Atomic number – the number of protons in an atom Atomic Mass – the mass of the protons and neutrons Mass Number – the number of protons and neutrons ...
... $400 Answer from Model of the Atom Atomic number – the number of protons in an atom Atomic Mass – the mass of the protons and neutrons Mass Number – the number of protons and neutrons ...
Development of atomic model
... actually able to calculate the distance between the nucleus and first energy shell and concluded that all shells are at fixed distances from the nucleus. He said that atoms absorb or give off energy when the electrons move from one shell to another. At this point, it was a commonly accepted model th ...
... actually able to calculate the distance between the nucleus and first energy shell and concluded that all shells are at fixed distances from the nucleus. He said that atoms absorb or give off energy when the electrons move from one shell to another. At this point, it was a commonly accepted model th ...
The Atom
... What are atoms? • An atom is the smallest particle into which an element can be divided and still be the same substance. • In 1808, John Dalton published an atomic theory that said all atoms of a particular element are identical. • Dalton also said that atoms of an element differ from atoms of other ...
... What are atoms? • An atom is the smallest particle into which an element can be divided and still be the same substance. • In 1808, John Dalton published an atomic theory that said all atoms of a particular element are identical. • Dalton also said that atoms of an element differ from atoms of other ...
In actual laboratories, isotopes in a sample can be
... For example, atoms with 17 protons are chlorine atoms. However, not all chlorine atoms have the same number of neutrons. Atoms of the same element (i.e., with same number of protons) that have a different number of neutrons are called isotopes. One isotope of chlorine has 18 neutrons and a different ...
... For example, atoms with 17 protons are chlorine atoms. However, not all chlorine atoms have the same number of neutrons. Atoms of the same element (i.e., with same number of protons) that have a different number of neutrons are called isotopes. One isotope of chlorine has 18 neutrons and a different ...
Electron configuration
... Physicists and chemists use a standard notation to indicate the electron configurations of atoms and molecules. For atoms, the notation consists of a sequence of atomic orbital labels (e.g. for phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each orbital (or set ...
... Physicists and chemists use a standard notation to indicate the electron configurations of atoms and molecules. For atoms, the notation consists of a sequence of atomic orbital labels (e.g. for phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each orbital (or set ...
4.Chemical bonding and Molecular Structure
... c) The expanded octet : Elements in and beyond the third period of the periodic table have, apart from 3s and 3p orbitals, 3d orbitals also available for bonding. In a number of compounds of these elements there are more than eight valence electrons around the central atom. This is termed as the exp ...
... c) The expanded octet : Elements in and beyond the third period of the periodic table have, apart from 3s and 3p orbitals, 3d orbitals also available for bonding. In a number of compounds of these elements there are more than eight valence electrons around the central atom. This is termed as the exp ...
Atomic Theory: History of the Atom
... Why is atomic mass of carbon given as 12.011 amu instead of as 12 amu? Atomic masses shown on periodic table are average atomic masses taking into account the different isotopes of each element and their percent abundances. Isotopes are atoms of the same element but with a different mass. These isot ...
... Why is atomic mass of carbon given as 12.011 amu instead of as 12 amu? Atomic masses shown on periodic table are average atomic masses taking into account the different isotopes of each element and their percent abundances. Isotopes are atoms of the same element but with a different mass. These isot ...
Unit 3.pmd
... Each question has one correct option. Choose the correct option. In the modern periodic table, elements are arranged in order of increasing atomic numbers which is related to the electronic configuration. Depending upon the type of orbitals receiving the last electron, the elements in the periodic t ...
... Each question has one correct option. Choose the correct option. In the modern periodic table, elements are arranged in order of increasing atomic numbers which is related to the electronic configuration. Depending upon the type of orbitals receiving the last electron, the elements in the periodic t ...
chap03 Matter and Atomic Structure
... Covalent Bonds One way in which atoms fill their outermost energy levels is by sharing electrons. For example, two hydrogen atoms can combine with each other by sharing electrons. Individual atoms of hydrogen each have just one electron. Each atom becomes more stable when it shares its electron with ...
... Covalent Bonds One way in which atoms fill their outermost energy levels is by sharing electrons. For example, two hydrogen atoms can combine with each other by sharing electrons. Individual atoms of hydrogen each have just one electron. Each atom becomes more stable when it shares its electron with ...
These are the periodic trends we need to know
... of these magnificent brains, there is quite a marvelous story to tell. All right, you must first understand the times, and what was, and was not, known. Around 1908, it is pretty well established that (there are): negative particles called electrons positive particles called alpha particles el ...
... of these magnificent brains, there is quite a marvelous story to tell. All right, you must first understand the times, and what was, and was not, known. Around 1908, it is pretty well established that (there are): negative particles called electrons positive particles called alpha particles el ...
Chemistry Chapter 3
... 2. Magnesium will react with bromine to form a compound called magnesium bromide, MgBr2. If 10.00 g of magnesium is mixed with 65.75 g of bromine and allowed to react, you would expect the mass of the products of the reaction to be? A. 75.75 g. B. less than 75.75 g. C. more than 75.75 g. D. unpredic ...
... 2. Magnesium will react with bromine to form a compound called magnesium bromide, MgBr2. If 10.00 g of magnesium is mixed with 65.75 g of bromine and allowed to react, you would expect the mass of the products of the reaction to be? A. 75.75 g. B. less than 75.75 g. C. more than 75.75 g. D. unpredic ...
The Structure of an Atom
... Dalton’s Atomic Theory • All matter is composed of extremely small particles called atoms. • All atoms of a given element are identical. • Atoms cannot be created, divided into smaller particles, or destroyed. (This part proven wrong) • Different atoms combine in simple whole number ratios to form ...
... Dalton’s Atomic Theory • All matter is composed of extremely small particles called atoms. • All atoms of a given element are identical. • Atoms cannot be created, divided into smaller particles, or destroyed. (This part proven wrong) • Different atoms combine in simple whole number ratios to form ...
5.3 Representative Groups PPT
... n Relate the number of valence electrons to groups in the periodic table and to properties of elements in those groups. n Predict the reactivity of some elements based on their locations within a group. n Identify some properties of common A group elements. ...
... n Relate the number of valence electrons to groups in the periodic table and to properties of elements in those groups. n Predict the reactivity of some elements based on their locations within a group. n Identify some properties of common A group elements. ...
4.1 Section Assessment
... Thomson thought the atom was a mass of positive charge with negative electrons embedded in its outer surface. Rutherford’s model didn’t envision the atom being a big ball of positive charge, but, rather, a tiny speck of positive charge in the middle of an almost perfectly empty region of space. Ruth ...
... Thomson thought the atom was a mass of positive charge with negative electrons embedded in its outer surface. Rutherford’s model didn’t envision the atom being a big ball of positive charge, but, rather, a tiny speck of positive charge in the middle of an almost perfectly empty region of space. Ruth ...
BS5-Ch 2.
... energy to overcome the attractions between particles Molecular compounds have weak attractions between particles and so tend to have low melting points Many molecular compounds are gases at room ...
... energy to overcome the attractions between particles Molecular compounds have weak attractions between particles and so tend to have low melting points Many molecular compounds are gases at room ...
Atomic Theory reading packet
... As an example, the principle of uncertainty says that it is impossible to describe with perfect accuracy both the position and the motion of an object. In other words, you might be able to say very accurately where an electron is located in an atom, but to do so reduces the accuracy with which you c ...
... As an example, the principle of uncertainty says that it is impossible to describe with perfect accuracy both the position and the motion of an object. In other words, you might be able to say very accurately where an electron is located in an atom, but to do so reduces the accuracy with which you c ...
- Dr.Divan Fard
... is called a group of elements. • A group contains elements with similar chemical and physical properties. • Each group is identified by a group number at the top of the column. • The representative elements have group numbers of 1A – 8A. The transition elements use the letter “B.” ...
... is called a group of elements. • A group contains elements with similar chemical and physical properties. • Each group is identified by a group number at the top of the column. • The representative elements have group numbers of 1A – 8A. The transition elements use the letter “B.” ...