Atoms and Periodic Table
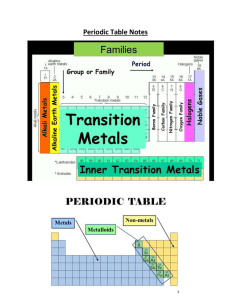
... Actinide Series • Fifteen elements that start with actinium (Ac) at atomic number 89 and finishing up with lawrencium (Lr) at number 103. • They are all radioactive and some are not found in nature. ...
... Actinide Series • Fifteen elements that start with actinium (Ac) at atomic number 89 and finishing up with lawrencium (Lr) at number 103. • They are all radioactive and some are not found in nature. ...
Introduction_to_Geochemistry_Pre-Lecture_Quiz
... detach the loosest electron from atoms of that element. (e) All alkali metals have similar chemical properties. (f) Alkali earths have one electron in the outer shell. (g) Electronegativity is the amount of negative charge on an atom. (h) Ca has a valency of 2. (i) True ionic bonds are unknown and a ...
... detach the loosest electron from atoms of that element. (e) All alkali metals have similar chemical properties. (f) Alkali earths have one electron in the outer shell. (g) Electronegativity is the amount of negative charge on an atom. (h) Ca has a valency of 2. (i) True ionic bonds are unknown and a ...
File
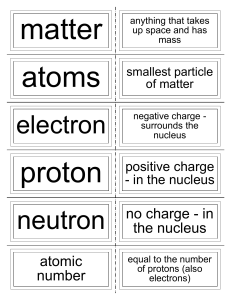
... atom: The smallest particles that make up matter. proton: a subatomic particle that has a positive charge and that is located in the nucleus of an atom. (The number of protons in the nucleus is the atomic number, which determines the identity of an element.) neutron: a subatomic particle that has no ...
... atom: The smallest particles that make up matter. proton: a subatomic particle that has a positive charge and that is located in the nucleus of an atom. (The number of protons in the nucleus is the atomic number, which determines the identity of an element.) neutron: a subatomic particle that has no ...
Period Table, valence Electrons and Ion Notes
... *Since there are 11 p+ and 10 e-, there are more +’s than –‘s creating a + change for the atom. Example: ...
... *Since there are 11 p+ and 10 e-, there are more +’s than –‘s creating a + change for the atom. Example: ...
CHEMISTRY TERMS Period: Elements in the same horizontal row
... Period: Elements in the same horizontal row with the same ground state energy level. Periodic Law: Elements list in order of their atomic numbers that fall into reoccurring groups. Ionic Radius: the radius of an atom’s ion, measured by the distance between ions in a crystal lattice. Atomic Radius: o ...
... Period: Elements in the same horizontal row with the same ground state energy level. Periodic Law: Elements list in order of their atomic numbers that fall into reoccurring groups. Ionic Radius: the radius of an atom’s ion, measured by the distance between ions in a crystal lattice. Atomic Radius: o ...
Quiz review
... Horizontal rows of the periodic table are called this. Vertical columns of the periodic table are called ‘groups’ or this. Which element in period 3 has 6 valence electrons? Which element in period 5 has only 1 electron in its 5s sublevel? Which element in period 3 has a full octet? What family of e ...
... Horizontal rows of the periodic table are called this. Vertical columns of the periodic table are called ‘groups’ or this. Which element in period 3 has 6 valence electrons? Which element in period 5 has only 1 electron in its 5s sublevel? Which element in period 3 has a full octet? What family of e ...
Periodicity review handout
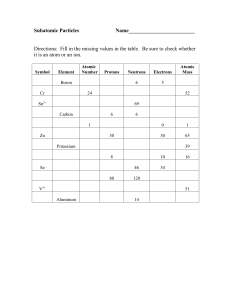
... 12. Consider a neutral atom with 53 protons and 74 neutrons to answer the following questions. a. ...
... 12. Consider a neutral atom with 53 protons and 74 neutrons to answer the following questions. a. ...
Intro to Atoms Clicker Questions 1. "atomos" means? 2. Atoms of one
... 2. Atoms of one kind of element _______ be changed into a different element with ordinary chemical means. (can, can’t) 3. Every compound is composed of atoms of different elements combined how? 4. In Thompson's model of the atom, the negatively charged electrons were located how in the atom? 5. In R ...
... 2. Atoms of one kind of element _______ be changed into a different element with ordinary chemical means. (can, can’t) 3. Every compound is composed of atoms of different elements combined how? 4. In Thompson's model of the atom, the negatively charged electrons were located how in the atom? 5. In R ...
The Atom and how it is organized - Cashmere
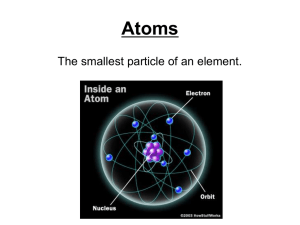
... The atoms of all elements are made up of a central nucleus with orbiting electrons. ◦ A nucleus is made up of positively charged PROTONS and neutral NEUTRONS. ◦ ELECTRONS are negatively charged and orbit around the nucleus. ...
... The atoms of all elements are made up of a central nucleus with orbiting electrons. ◦ A nucleus is made up of positively charged PROTONS and neutral NEUTRONS. ◦ ELECTRONS are negatively charged and orbit around the nucleus. ...
Homework Geochem Test Review
... 12. What part of an atom is negative? _________ What part is positive? _________ What part is neutral? ___________ 13. What is the atomic mass? Why don’t we count the electrons when determining the ...
... 12. What part of an atom is negative? _________ What part is positive? _________ What part is neutral? ___________ 13. What is the atomic mass? Why don’t we count the electrons when determining the ...
atomic number - Thomas C. Cario Middle School
... The periodic table is a chart containing information about the atoms that make up all matter. An element is a substance made up of only one type of atom. The atomic number of an atom is equal to the number of protons in its nucleus. The number of electrons surrounding the nucleus of an atom is equal ...
... The periodic table is a chart containing information about the atoms that make up all matter. An element is a substance made up of only one type of atom. The atomic number of an atom is equal to the number of protons in its nucleus. The number of electrons surrounding the nucleus of an atom is equal ...
Atomic and Molecular Structure – Standard 1 Review
... 1a.3 What does an element’s Mass Number (Atomic Mass) tell you about that element? ...
... 1a.3 What does an element’s Mass Number (Atomic Mass) tell you about that element? ...
Chemical Basis of Life
... 1- Also for example water is a liquid at room temperature, whereas both hydrogen and oxygen are gases at room temperature 2 - Some compounds are quite simple others are very complex, the important thing to remember is that a compound is always in a fixed ratio ...
... 1- Also for example water is a liquid at room temperature, whereas both hydrogen and oxygen are gases at room temperature 2 - Some compounds are quite simple others are very complex, the important thing to remember is that a compound is always in a fixed ratio ...
Mr. Trachtenberg`s Big Chemistry Test Review Part I of III 20pts
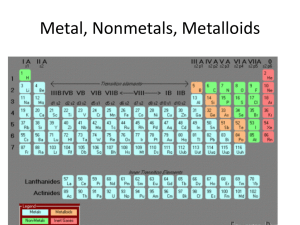
... 11.) Elements in the upper right-‐hand corner of the Periodic Table have the highest electronegativies and ionization energies. 12.) Moving down a group atoms get larger 13.) Moving from l ...
... 11.) Elements in the upper right-‐hand corner of the Periodic Table have the highest electronegativies and ionization energies. 12.) Moving down a group atoms get larger 13.) Moving from l ...
Ions and isotopes
... • An atom usually has a neutral charge. That means it has the same number of protons as electrons • Remember, a proton has a positive charge and an electron has a negative charge ...
... • An atom usually has a neutral charge. That means it has the same number of protons as electrons • Remember, a proton has a positive charge and an electron has a negative charge ...