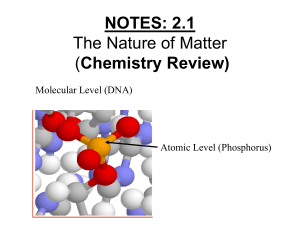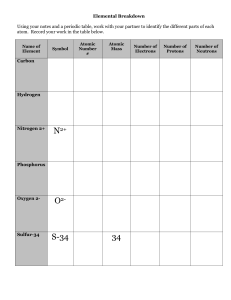
L.O.
... I have some understanding but I need to revise this some more I don’t know this or I need help because I don’t understand it ...
... I have some understanding but I need to revise this some more I don’t know this or I need help because I don’t understand it ...
Learning Standards vocab chemical basis and molecules of life 09
... Demonstrate how carbon atoms form four covalent bonds to make large molecules. Identify the functions of these molecules (e.g., plant and animal tissue, polymers, sources of food and nutrition, fossil fuels). Describe at least three chemical reactions of particular importance to humans (e.g., bu ...
... Demonstrate how carbon atoms form four covalent bonds to make large molecules. Identify the functions of these molecules (e.g., plant and animal tissue, polymers, sources of food and nutrition, fossil fuels). Describe at least three chemical reactions of particular importance to humans (e.g., bu ...
Atoms, Bonding, and the Periodic Table Electron Dot Diagrams
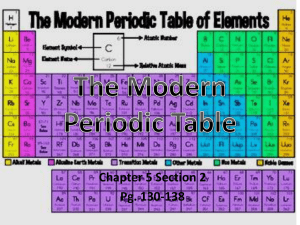
... Periodic Table of the Elements The periodic table is arranged in order of increasing atomic number. The number of valence electrons also increases from left to right across a period. What is the number of valence electrons for each group? ...
... Periodic Table of the Elements The periodic table is arranged in order of increasing atomic number. The number of valence electrons also increases from left to right across a period. What is the number of valence electrons for each group? ...
Chemistry Notes
... sodium, magnesium, aluminum, silicon, phosphorous, sulfur, chlorine, argon, potassium, calcium, iron, copper, zinc, bromine, silver, iodine, gold, lead, mercury, radon. Day 3 99% of the atoms mass in the nucleus The energy of the atom in the electron shells Most of an atom empty space ...
... sodium, magnesium, aluminum, silicon, phosphorous, sulfur, chlorine, argon, potassium, calcium, iron, copper, zinc, bromine, silver, iodine, gold, lead, mercury, radon. Day 3 99% of the atoms mass in the nucleus The energy of the atom in the electron shells Most of an atom empty space ...
20161025140773
... and not very useful because the samples of matter that scientists work with contain trillions of atoms – To have a convenient way to compare the masses of atoms, scientists chose one isotope to serve as a standard – An atomic mass unit (amu) is defined as one twelfth the mass of a carbon-12 atom ...
... and not very useful because the samples of matter that scientists work with contain trillions of atoms – To have a convenient way to compare the masses of atoms, scientists chose one isotope to serve as a standard – An atomic mass unit (amu) is defined as one twelfth the mass of a carbon-12 atom ...
CPA Study Guide for Chapter 6 Test The Periodic Table Know the
... Know the difference between Mendeleev’s organization of the elements versus the modern organization of the Periodic Table Know and be able to apply the Periodic Law Know the names of families of elements: alkali metals, alkaline earths, halogens, noble gases, metalloids and transition elements Defin ...
... Know the difference between Mendeleev’s organization of the elements versus the modern organization of the Periodic Table Know and be able to apply the Periodic Law Know the names of families of elements: alkali metals, alkaline earths, halogens, noble gases, metalloids and transition elements Defin ...
and the atomic
... 6 protons, 8 (14 - 6) neutrons, 6 electrons How many protons, neutrons, and electrons are in-6 protons, 5 (11 - 6) neutrons, 6 electrons ...
... 6 protons, 8 (14 - 6) neutrons, 6 electrons How many protons, neutrons, and electrons are in-6 protons, 5 (11 - 6) neutrons, 6 electrons ...
Worksheet
... 19. __________ He deduced the relationship between the energy and frequency of radiation. 20. __________ He proposed that light could be described as quanta of energy that behave as particles. ...
... 19. __________ He deduced the relationship between the energy and frequency of radiation. 20. __________ He proposed that light could be described as quanta of energy that behave as particles. ...
chapter 4 crossword pre-ap
... 21. An electron that is found in the outermost shell of an atom and that determines the atom's chemical properties. 22. Gold is in period __. 23. Sodium has ___ valence electron. 24. Can be hammered or rolled into sheets. 26. Half the distance from center to center of two like atoms that are bonded ...
... 21. An electron that is found in the outermost shell of an atom and that determines the atom's chemical properties. 22. Gold is in period __. 23. Sodium has ___ valence electron. 24. Can be hammered or rolled into sheets. 26. Half the distance from center to center of two like atoms that are bonded ...
BC1 Atoms Unit Standards
... of the protons, neutrons, and electrons in an atom of an element. 2a For a given element, determine the number of protons 2b When given a number of protons, identify the element name and symbol 2c Identify the number of neutrons in an atom from atomic number and mass number 2d Identify the number of ...
... of the protons, neutrons, and electrons in an atom of an element. 2a For a given element, determine the number of protons 2b When given a number of protons, identify the element name and symbol 2c Identify the number of neutrons in an atom from atomic number and mass number 2d Identify the number of ...
- Priddy ISD
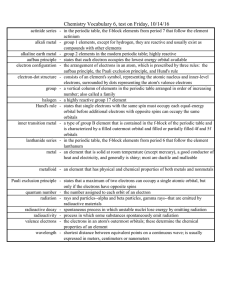
... aufbau principle - states that each electron occupies the lowest energy orbital available electron configuration - the arrangement of electrons in an atom, which is prescribed by three rules: the aufbau principle, the Pauli exclusion principle, and Hund's rule electron-dot structure - consists of an ...
... aufbau principle - states that each electron occupies the lowest energy orbital available electron configuration - the arrangement of electrons in an atom, which is prescribed by three rules: the aufbau principle, the Pauli exclusion principle, and Hund's rule electron-dot structure - consists of an ...
Chemistry Study Guide - Atomic structure and the Periodic Table 2010
... a. What is the structure of the atom and how are protons, neutrons, and electrons arranged b. How can you use the periodic table to identify elements in simple compounds. 7. The organization of the periodic table is based on the properties of the elements and reflects the structure of atoms. As a ba ...
... a. What is the structure of the atom and how are protons, neutrons, and electrons arranged b. How can you use the periodic table to identify elements in simple compounds. 7. The organization of the periodic table is based on the properties of the elements and reflects the structure of atoms. As a ba ...
Ch. 4: Atoms and the Periodic Table – Study Guide
... The first person who suggested that matter was made up of atoms was the Greek philosopher Democritus. The word atom comes from the Greek word that means “unable to be divided.” Dalton’s atomic theory stated that every element was made of atoms that could not be subdivided, atoms of the same element ...
... The first person who suggested that matter was made up of atoms was the Greek philosopher Democritus. The word atom comes from the Greek word that means “unable to be divided.” Dalton’s atomic theory stated that every element was made of atoms that could not be subdivided, atoms of the same element ...
Unit 4 Review - Davis
... Modern Periodic Law – The properties of the elements are a periodic function of their atomic numbers. The statement that the physical and chemical properties of the elements repeat in a regular pattern when they are arranged in order of increasing atomic number is known as the periodic law. Octet Ru ...
... Modern Periodic Law – The properties of the elements are a periodic function of their atomic numbers. The statement that the physical and chemical properties of the elements repeat in a regular pattern when they are arranged in order of increasing atomic number is known as the periodic law. Octet Ru ...
Atom
... 1904: J.J.Thompson discovered electrons & proposed the “plum pudding model” 1911: Earnest Rutherford discovered the nucleus. 1913: Neils Bohr proposed that electrons orbit with electrostic forces rather than gravity. the “planetary model” 1926: Erwin Schrodinger analyzed electron orbits from a geome ...
... 1904: J.J.Thompson discovered electrons & proposed the “plum pudding model” 1911: Earnest Rutherford discovered the nucleus. 1913: Neils Bohr proposed that electrons orbit with electrostic forces rather than gravity. the “planetary model” 1926: Erwin Schrodinger analyzed electron orbits from a geome ...
C2- Topic 1: Atomic structure and the periodic table. Assessable
... - arranged the elements, known at that time, in a periodic table by using properties of these elements and their compounds - used his table to predict the existence and properties of some elements not then discovered ...
... - arranged the elements, known at that time, in a periodic table by using properties of these elements and their compounds - used his table to predict the existence and properties of some elements not then discovered ...
C2 Topic 1 Can Do Sheet
... a arranged the elements, known at that time, in a periodic table by using properties of these elements and their compounds b used his table to predict the existence and properties of some elements not then discovered 1.2 Classify elements as metals or non-metals according to their position in the pe ...
... a arranged the elements, known at that time, in a periodic table by using properties of these elements and their compounds b used his table to predict the existence and properties of some elements not then discovered 1.2 Classify elements as metals or non-metals according to their position in the pe ...
The Periodic Table Chemistry – Leaving Cert Quick Notes
... Davy isolated potassium and sodium from their hydroxide compounds. Henry Moseley used the concept of atomic number to derive another definition for an element: a substance all of those whose atoms have the same atomic number. Dobereiner suggested elements of similar properties could be arranged in g ...
... Davy isolated potassium and sodium from their hydroxide compounds. Henry Moseley used the concept of atomic number to derive another definition for an element: a substance all of those whose atoms have the same atomic number. Dobereiner suggested elements of similar properties could be arranged in g ...