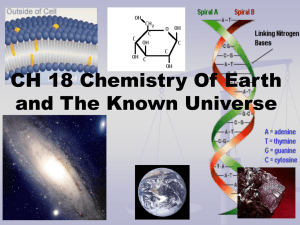
1. In what order did Mendeleev arrange the elements in his periodic
... b) decreasing atomic number c) increasing number of neutrons d) increasing atomic weight 2. What family of elements was unknown when Mendeleev created the periodic table? a) noble gases b) alkali metals c) alkaline earth metals d) halogens 3. Mendeleev predicted the existence of which then unknown e ...
... b) decreasing atomic number c) increasing number of neutrons d) increasing atomic weight 2. What family of elements was unknown when Mendeleev created the periodic table? a) noble gases b) alkali metals c) alkaline earth metals d) halogens 3. Mendeleev predicted the existence of which then unknown e ...
Atomic Structure/Electrons
... a. contains at least one proton, neutron and electron. b. retains the chemical identity of the element. c. can carry an electrical charge. d. is affected in a cathode ray tube. 7. The electrical charges in an atom are located: a. only in the nucleus b. on protons and neutrons c. on protons and elect ...
... a. contains at least one proton, neutron and electron. b. retains the chemical identity of the element. c. can carry an electrical charge. d. is affected in a cathode ray tube. 7. The electrical charges in an atom are located: a. only in the nucleus b. on protons and neutrons c. on protons and elect ...
Chapter 2
... • “atomos” – later became atom – small, indivisible particles • Dalton’s Atomic Theory – • Each element made up of atoms • Atoms of same element same; different element different • Compounds form from combinations of elements in definite proportions. • Chemical reactions involve rearrangements of at ...
... • “atomos” – later became atom – small, indivisible particles • Dalton’s Atomic Theory – • Each element made up of atoms • Atoms of same element same; different element different • Compounds form from combinations of elements in definite proportions. • Chemical reactions involve rearrangements of at ...
CLASS TEST NAME Class IIB Date ______ 1 .Which atomic
... 9. _______________________________________ are atoms of the same element that have different numbers of neutrons. 10.The atomic number of an element tells you the number of _________________________________________. 11. An ion is a form of an atom with a different number of__________________________ ...
... 9. _______________________________________ are atoms of the same element that have different numbers of neutrons. 10.The atomic number of an element tells you the number of _________________________________________. 11. An ion is a form of an atom with a different number of__________________________ ...
Bill Nye Science Video – Atoms 1. Atom is from a Greek word
... C. neutron 3. The flow of electrons from one atom to another is ... A. B. C. D. ...
... C. neutron 3. The flow of electrons from one atom to another is ... A. B. C. D. ...
Quiz: The Atom (Open Notes)
... 10. T or F A neutron carries a positive charge. 11. T or F A proton has a charge that is equal in force but opposite in charge to each electron. 12. T or F Protons and electrons are about equal in mass. 13. T or F The mass of an atom depends on the number of protons and neutrons in its nucleus. 14. ...
... 10. T or F A neutron carries a positive charge. 11. T or F A proton has a charge that is equal in force but opposite in charge to each electron. 12. T or F Protons and electrons are about equal in mass. 13. T or F The mass of an atom depends on the number of protons and neutrons in its nucleus. 14. ...
Chapter 18 Notes
... Proton- P+ positive charged - in nucleus Number of P+ distinguishes one atom from another Made of 2 up quarks (+2/3 charge) and 1 down ...
... Proton- P+ positive charged - in nucleus Number of P+ distinguishes one atom from another Made of 2 up quarks (+2/3 charge) and 1 down ...
Understanding the Atom GN
... When atoms of the same element have different numbers of neutrons they are called ____________________. Isotope – ________________________________________________________________________ Most elements have ______________________ isotopes. Mass Number - ________________________________________ ...
... When atoms of the same element have different numbers of neutrons they are called ____________________. Isotope – ________________________________________________________________________ Most elements have ______________________ isotopes. Mass Number - ________________________________________ ...
Chapter 7 Review Sheet
... 5. It would require more energy to remove an electron from Az than from any other element. 6. Jq has one more valence electron than Dw but one less valence electron than Gt. 7. The sizes of the 3 isoelectronic species are: Cx+ < Hs < By–. 8. Ev and Kp both lose the same number of electrons when they ...
... 5. It would require more energy to remove an electron from Az than from any other element. 6. Jq has one more valence electron than Dw but one less valence electron than Gt. 7. The sizes of the 3 isoelectronic species are: Cx+ < Hs < By–. 8. Ev and Kp both lose the same number of electrons when they ...
Setting up Programmable PRS Keypad as Fixed ID Keypads
... Parser06 example6
The identity of an element is determined by the number of…
Q
Protons
Neutrons
Electrons
A molecule may consist of one atom.
Q
True
False
Molybdenum has atomic number 42. Its molar mass is 95.94 grams/mole. How many neutrons does
the most common isotope have?
Q
...
... Parser06 example
Ch. 14 Test Review
... Ch. 14 Test Review CHEMICAL PERIODICITY COMPLETION decreases increases (3) groups (2) periods transition metals ionization energy atomic # noble gases representative electronegativity The periodic table organizes the elements into vertical ____________ and horizontal ____________ in order of increas ...
... Ch. 14 Test Review CHEMICAL PERIODICITY COMPLETION decreases increases (3) groups (2) periods transition metals ionization energy atomic # noble gases representative electronegativity The periodic table organizes the elements into vertical ____________ and horizontal ____________ in order of increas ...
Chemistry
... one atom of the element. Atomic Number – the number of protons contained in each nucleus of its atoms of the element. Period – a horizontal row in the periodic table. Group – a vertical column on the periodic table. Reactivity – describes how likely an element is to form bonds with other elements. V ...
... one atom of the element. Atomic Number – the number of protons contained in each nucleus of its atoms of the element. Period – a horizontal row in the periodic table. Group – a vertical column on the periodic table. Reactivity – describes how likely an element is to form bonds with other elements. V ...
Chapter 4 Study Guide Physical Science 1. The word atom comes
... 2. Halogens are very reactive elements located in Group _______of the periodic table. 3. The nucleus of an atom has a(n) ____________________ electric charge. 4. Carbon is found in group ______ of the periodic table. 5. Bohr’s model of the atom compares electrons to ____________________. 6. Elements ...
... 2. Halogens are very reactive elements located in Group _______of the periodic table. 3. The nucleus of an atom has a(n) ____________________ electric charge. 4. Carbon is found in group ______ of the periodic table. 5. Bohr’s model of the atom compares electrons to ____________________. 6. Elements ...
Elements and Atoms - Portola Middle School
... neutron or proton. Protons should have a + or P written on them. Neutrons should be blank or have an N. In a circle around the nucleus are the electrons. Electrons should have a minus sign or an e. ...
... neutron or proton. Protons should have a + or P written on them. Neutrons should be blank or have an N. In a circle around the nucleus are the electrons. Electrons should have a minus sign or an e. ...
Name
... the elements that make up the Earth. However, the inhabitants of the alien planet have different names and symbols for them. Since the alien scientists do not know the names of our elements, they have radioed the following data on the known properties of the elements. They have not yet radioed the i ...
... the elements that make up the Earth. However, the inhabitants of the alien planet have different names and symbols for them. Since the alien scientists do not know the names of our elements, they have radioed the following data on the known properties of the elements. They have not yet radioed the i ...
Atomic Structure
... Ground State Electron Configuration of Sulfur: 2 – 8 – 6 Excited State Sulfur: 2 – 8 – 5 – 1 THE WEIGHTED AVERAGE OF NATURALLY OCCURING ISOTOPES Weighted average = (mass of isotope #1) x (% abundance) + (mass #2) x (%) + … ...
... Ground State Electron Configuration of Sulfur: 2 – 8 – 6 Excited State Sulfur: 2 – 8 – 5 – 1 THE WEIGHTED AVERAGE OF NATURALLY OCCURING ISOTOPES Weighted average = (mass of isotope #1) x (% abundance) + (mass #2) x (%) + … ...
Atom - Sites
... atoms join together chemically. •Combinations of two or more different elements are called compounds. •All compounds are molecules but not all molecules are compounds. (ex. H2O vs. O2) •Molecules can also join together to form larger molecules. •Many, many repeating small molecules joined together f ...
... atoms join together chemically. •Combinations of two or more different elements are called compounds. •All compounds are molecules but not all molecules are compounds. (ex. H2O vs. O2) •Molecules can also join together to form larger molecules. •Many, many repeating small molecules joined together f ...
Chapter 4.1
... 2. Electrons – negative particles on orbits around the nucleus -charge is -1 -# electons = # protons= atomic # 3. Neutrons – neutral particles in the nucleus -charge is 0 -#neutrons= mass-atomic # ...
... 2. Electrons – negative particles on orbits around the nucleus -charge is -1 -# electons = # protons= atomic # 3. Neutrons – neutral particles in the nucleus -charge is 0 -#neutrons= mass-atomic # ...
September 28th Notes
... Atomic Structure Element: matter that is composed of one type of atom. Elements are abbreviated in scientific shorthand- either a letter or a pair of letters called a chemical symbol. Ex- Aluminum =Al Copper=Cu Atom- smallest piece of matter that still has the properties of the element. Protons- pos ...
... Atomic Structure Element: matter that is composed of one type of atom. Elements are abbreviated in scientific shorthand- either a letter or a pair of letters called a chemical symbol. Ex- Aluminum =Al Copper=Cu Atom- smallest piece of matter that still has the properties of the element. Protons- pos ...
In the space provided, write the letter of the term or phrase that best
... ______ 5. Nonmetallic elements in Group 17 that react with most metals to form salts are a. alkali metals. b. halogens. c. lanthanides. d. noble gases. ______ 6. The outer-level electron configuration of a neutral alkaline-earth metal atom consists of a. one electron in the s orbital. b. two electro ...
... ______ 5. Nonmetallic elements in Group 17 that react with most metals to form salts are a. alkali metals. b. halogens. c. lanthanides. d. noble gases. ______ 6. The outer-level electron configuration of a neutral alkaline-earth metal atom consists of a. one electron in the s orbital. b. two electro ...