Biol160 Chemistry The Basic Chemistry of Life In order to
... magnesium (Mg) atom. If it were possible for that atom to lose one proton, so that it only had 11, then we would be dealing with the element sodium (Na). The number of neutrons and electrons present in an atom is often the same as the number of protons. For example, a typical helium (He) atom has 2 ...
... magnesium (Mg) atom. If it were possible for that atom to lose one proton, so that it only had 11, then we would be dealing with the element sodium (Na). The number of neutrons and electrons present in an atom is often the same as the number of protons. For example, a typical helium (He) atom has 2 ...
13.2 Chemical Formulas
... What is a chemical formula? Chemical formulas have two important parts: chemical symbols for the elements in the compound and subscripts that tell how many atoms of each element are needed to form the compound. The chemical formula for water, H2O, tells us that a water molecule is made of the elemen ...
... What is a chemical formula? Chemical formulas have two important parts: chemical symbols for the elements in the compound and subscripts that tell how many atoms of each element are needed to form the compound. The chemical formula for water, H2O, tells us that a water molecule is made of the elemen ...
Name: Period:______ Table Number:______
... 86. The names of the different energy levels, shells or orbitals of electrons which compose the electron cloud of an atom, starting with the shell of electrons which is closest to the nucleus are the ___________ shell, ___________ shell, __________ shell, and __________ shell. 87. The maximum number ...
... 86. The names of the different energy levels, shells or orbitals of electrons which compose the electron cloud of an atom, starting with the shell of electrons which is closest to the nucleus are the ___________ shell, ___________ shell, __________ shell, and __________ shell. 87. The maximum number ...
(a) Atoms - Warren County Schools
... more of the entire unit it appears in front of. The coefficient used in this example shows that, in the left reactant, there are 4 hydrogen, and in the product, there are 4 hydrogen and 2 oxygen. • The coefficient does not effect the oxygen in the reactant because it is not a compound with hydrogen ...
... more of the entire unit it appears in front of. The coefficient used in this example shows that, in the left reactant, there are 4 hydrogen, and in the product, there are 4 hydrogen and 2 oxygen. • The coefficient does not effect the oxygen in the reactant because it is not a compound with hydrogen ...
0321813545_08_final
... attraction for each electron. In anions, there is a lower average attraction between nucleus and electrons compared to the neutral atom. Conceptual Connection 8.5 Ions, Isotopes, and Atomic ...
... attraction for each electron. In anions, there is a lower average attraction between nucleus and electrons compared to the neutral atom. Conceptual Connection 8.5 Ions, Isotopes, and Atomic ...
Thomson`s Model of the Atom
... with electrons orbiting the nucleus similar to the planets revolving around the sun. He determined that orbits of electrons depend on their energy, and that electrons can jump from one energy level to another; energy travels in discrete quantities. James Chadwick: In 1921, while working with E.S. ...
... with electrons orbiting the nucleus similar to the planets revolving around the sun. He determined that orbits of electrons depend on their energy, and that electrons can jump from one energy level to another; energy travels in discrete quantities. James Chadwick: In 1921, while working with E.S. ...
atoms
... with electrons orbiting the nucleus similar to the planets revolving around the sun. He determined that orbits of electrons depend on their energy, and that electrons can jump from one energy level to another; energy travels in discrete quantities. James Chadwick: In 1921, while working with E.S. ...
... with electrons orbiting the nucleus similar to the planets revolving around the sun. He determined that orbits of electrons depend on their energy, and that electrons can jump from one energy level to another; energy travels in discrete quantities. James Chadwick: In 1921, while working with E.S. ...
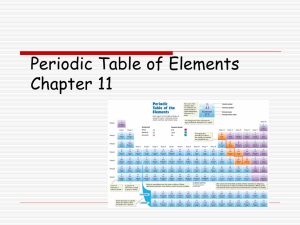
periodic table
... Significance of Avogadro’s Number There must be something special about the number 6.022 x 1023 (Avogadro’s number). The significance is as follows. Consider a collection of identical objects. The following relationship will apply. If one object has a mass of X amu… …then one mole of objects has a ...
... Significance of Avogadro’s Number There must be something special about the number 6.022 x 1023 (Avogadro’s number). The significance is as follows. Consider a collection of identical objects. The following relationship will apply. If one object has a mass of X amu… …then one mole of objects has a ...
Periodic Trends: Straw Lab
... Answer the following questions in your notebook: 1) In a sentence, describe the relationship between atomic number and the amount of ionization energy as you go down a group on the periodic table. 2) Based on your understanding of ionization energy and atomic size, explain why this trend makes sens ...
... Answer the following questions in your notebook: 1) In a sentence, describe the relationship between atomic number and the amount of ionization energy as you go down a group on the periodic table. 2) Based on your understanding of ionization energy and atomic size, explain why this trend makes sens ...
AtomMoleculeNaming_G1
... differences in particle size. Filtration usually involves separating a precipitate from solution. Crystallization: Separation is based upon differences in solubility of the components in a mixture. Distillation: Separation is based upon differences in volatility. Extraction: Separation is based upon ...
... differences in particle size. Filtration usually involves separating a precipitate from solution. Crystallization: Separation is based upon differences in solubility of the components in a mixture. Distillation: Separation is based upon differences in volatility. Extraction: Separation is based upon ...
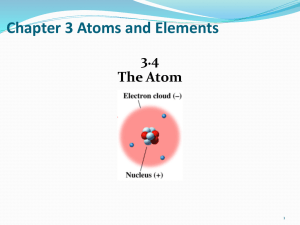
Chapter 3 Atoms and Elements
... A sample of naturally occurring sulfur contains several isotopes with the following abundances Isotope % abundance 32S ...
... A sample of naturally occurring sulfur contains several isotopes with the following abundances Isotope % abundance 32S ...
Period
... 1. _____________________________= one-half the distance between the nuclei of identical atoms joined in a molecule ...
... 1. _____________________________= one-half the distance between the nuclei of identical atoms joined in a molecule ...
Atoms and Atomic Theory
... electrons travel the nucleus in a fixed orbit. At times, electrons can move from one energy level to another. Charge-Cloud Model With the emergence of Werner Heisenberg's Uncertainty Principle, which states that it is impossible to know both the location and speed of an electron at the same time, sc ...
... electrons travel the nucleus in a fixed orbit. At times, electrons can move from one energy level to another. Charge-Cloud Model With the emergence of Werner Heisenberg's Uncertainty Principle, which states that it is impossible to know both the location and speed of an electron at the same time, sc ...
Inorganic Chemistry ELEMENTS AND
... Chemical Bond : Chemical bond may be defined as the attractive force that binds together the constituent atoms in a molecule. Following are some different types of chemical bonds which usually occur in various molecules. (i) Electrovalent bond (Ionic bond) : This type of bond is formed by transfer o ...
... Chemical Bond : Chemical bond may be defined as the attractive force that binds together the constituent atoms in a molecule. Following are some different types of chemical bonds which usually occur in various molecules. (i) Electrovalent bond (Ionic bond) : This type of bond is formed by transfer o ...
Placing Elements on the Periodic Table
... Lanthanides and Actinides Some are Radioactive The rare earths are silver, silverywhite, or gray metals. Conduct electricity ...
... Lanthanides and Actinides Some are Radioactive The rare earths are silver, silverywhite, or gray metals. Conduct electricity ...
File - LIVING THE CHEM LIFE
... ERNEST RUTHERFORD- he simply have discovered the proton or the positively charged particle inside the nucleus. In his atomic model, the atom is comprised of a single positive nucleus surrounded by negative orbiting electrons. Also, it suggests that most of the mass of the atoms are contained in the ...
... ERNEST RUTHERFORD- he simply have discovered the proton or the positively charged particle inside the nucleus. In his atomic model, the atom is comprised of a single positive nucleus surrounded by negative orbiting electrons. Also, it suggests that most of the mass of the atoms are contained in the ...
transcript for this video
... Periodic Table in terms of atomic mass and atomic number. And, finally, to the idea that we’re still - looking at the Periodic Table, looking at the transient relevance – to this day, trying to synthesise new and ever more complex elements. I really like this, but I thought the use of images could h ...
... Periodic Table in terms of atomic mass and atomic number. And, finally, to the idea that we’re still - looking at the Periodic Table, looking at the transient relevance – to this day, trying to synthesise new and ever more complex elements. I really like this, but I thought the use of images could h ...
pdf.format - San Diego Mesa College
... Elements are made from atoms having the same atomic number, protons Are all atoms of one particular atom the same or are they mixtures? 1) All atom nuclei for an element have the same number of protons. 2) Every atom in an element has the same number of protons & electrons. 3) However, elements are ...
... Elements are made from atoms having the same atomic number, protons Are all atoms of one particular atom the same or are they mixtures? 1) All atom nuclei for an element have the same number of protons. 2) Every atom in an element has the same number of protons & electrons. 3) However, elements are ...
Group 2 Elements
... elements down the group •know the reactions of the elements Mg to Ba in Group 2 with oxygen, chlorine and water •understand the formation of characteristic flame colours by Group 1 and 2 compounds in terms of electron transitions •know the flame colours for Groups 1 and 2 compounds •understand exper ...
... elements down the group •know the reactions of the elements Mg to Ba in Group 2 with oxygen, chlorine and water •understand the formation of characteristic flame colours by Group 1 and 2 compounds in terms of electron transitions •know the flame colours for Groups 1 and 2 compounds •understand exper ...
Chapter 5
... Example: Naturally occurring Cu consists of 2 isotopes. It is 69.1% 63Cu with a mass of 62.9 amu, and 30.9% 65Cu, which has a mass of 64.9 amu. Calculate the atomic weight of Cu to one decimal place. ...
... Example: Naturally occurring Cu consists of 2 isotopes. It is 69.1% 63Cu with a mass of 62.9 amu, and 30.9% 65Cu, which has a mass of 64.9 amu. Calculate the atomic weight of Cu to one decimal place. ...
Atomic theory
... 3. Atoms of different elements differ in their physical and chemical properties. 4. Atoms of different elements combine in simple, whole number ratios to form compounds. 5. In chemical reactions, atoms are combined, separated, or rearranged but never created, destroyed, or changed. ...
... 3. Atoms of different elements differ in their physical and chemical properties. 4. Atoms of different elements combine in simple, whole number ratios to form compounds. 5. In chemical reactions, atoms are combined, separated, or rearranged but never created, destroyed, or changed. ...
Introduction to Chemical Bonding
... The bond of Sodium and Fluorine is an example of Ionic bonding: electrons have been transferred in order for the atoms to have a full outer level. When an atom loses or gains electrons, it becomes what is called an ion. An ion is no longer neutrally charged because it has different numbers of proton ...
... The bond of Sodium and Fluorine is an example of Ionic bonding: electrons have been transferred in order for the atoms to have a full outer level. When an atom loses or gains electrons, it becomes what is called an ion. An ion is no longer neutrally charged because it has different numbers of proton ...
Grade 11 Unit 4 - Amazon Web Services
... great contributions to the development of our present-day atomic theory. Information on each scientist is taken from the “Atomic Pioneer Series.” United States Energy Research and Development Administration Technical Information Center Oak Ridge, TN 37830 The three-volume set is available from ERDA. ...
... great contributions to the development of our present-day atomic theory. Information on each scientist is taken from the “Atomic Pioneer Series.” United States Energy Research and Development Administration Technical Information Center Oak Ridge, TN 37830 The three-volume set is available from ERDA. ...
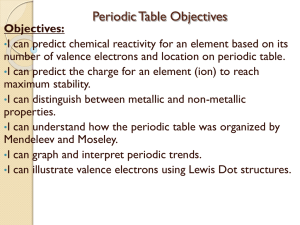
Objectives - Warren County Public Schools
... Periodic Table Worksheet-qts. 12-16 Objectives: •I can predict chemical reactivity for an element based on its number of valence electrons and location on periodic table. •I can predict the charge for an element to reach stability. •I can distinguish between metallic and non-metallic properties. ...
... Periodic Table Worksheet-qts. 12-16 Objectives: •I can predict chemical reactivity for an element based on its number of valence electrons and location on periodic table. •I can predict the charge for an element to reach stability. •I can distinguish between metallic and non-metallic properties. ...