Atomic Structure Powerpoints - Warren County Public Schools
... • I can recognize that the periodic table is organized by an element’s atomic number. • I can divide the elements in the periodic table into periods and groups. • I can identify and distinguish between metals, nonmetals, and metalloids on the periodic table. • I can determine if an atom is neutral o ...
... • I can recognize that the periodic table is organized by an element’s atomic number. • I can divide the elements in the periodic table into periods and groups. • I can identify and distinguish between metals, nonmetals, and metalloids on the periodic table. • I can determine if an atom is neutral o ...
The Chemical Context of Life
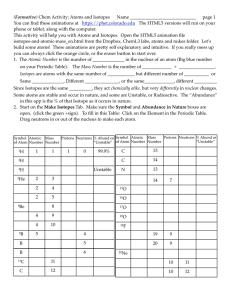
... Isotopes are two atoms of an element that differ in number of neutrons ...
... Isotopes are two atoms of an element that differ in number of neutrons ...
Structure and Bonding
... In 1865 Kekulé suggested that carbon chains can double back to form rings of atoms In 1874 Jacobus van’t Hoff and Joseph Le Bel proposed four atoms to which carbon is bonded sit at the corner of a regular tetrahedron ...
... In 1865 Kekulé suggested that carbon chains can double back to form rings of atoms In 1874 Jacobus van’t Hoff and Joseph Le Bel proposed four atoms to which carbon is bonded sit at the corner of a regular tetrahedron ...
Periodic Table - Derry Area School District
... periodic table. – The total number of valence electrons for an atom equals its group number. Page 55 ...
... periodic table. – The total number of valence electrons for an atom equals its group number. Page 55 ...
TEST-Atomic Structure
... ____ 13. Which statement about subatomic particles is NOT true? a. Protons and neutrons have almost the same mass. b. Protons and electrons have opposite charges. c. Unlike protons and electrons, neutrons have no charge. d. Protons and neutrons have the same charge. ____ 14. Which statement about s ...
... ____ 13. Which statement about subatomic particles is NOT true? a. Protons and neutrons have almost the same mass. b. Protons and electrons have opposite charges. c. Unlike protons and electrons, neutrons have no charge. d. Protons and neutrons have the same charge. ____ 14. Which statement about s ...
Chapter3 Solutions
... separate and the elements are gases. Another factor that contributes is the fact that the elements are relatively light, compared to iodine, for example. 11. Ionic compounds do not contain molecules, since one ion is bound to several other ions of opposite charge in a lattice. To speak of intermolec ...
... separate and the elements are gases. Another factor that contributes is the fact that the elements are relatively light, compared to iodine, for example. 11. Ionic compounds do not contain molecules, since one ion is bound to several other ions of opposite charge in a lattice. To speak of intermolec ...
Chapter 2 - Old Saybrook Public Schools
... Postulated the existence of negatively charged particles, that we now call electrons, using cathode-ray tubes. Determined the charge-to-mass ratio of an electron. The atom must also contain positive particles that balance exactly the negative charge carried by electrons. ...
... Postulated the existence of negatively charged particles, that we now call electrons, using cathode-ray tubes. Determined the charge-to-mass ratio of an electron. The atom must also contain positive particles that balance exactly the negative charge carried by electrons. ...
2.2 The Discovery of Atomic Structure
... Greek Philosophers: Can matter be subdivided into fundamental particles? Democritus (460–370 BC): All matter can be divided into indivisible atomos. Dalton: proposed atomic theory with the following postulates: • Elements are composed of atoms. • All atoms of an element are identical. • In chemical ...
... Greek Philosophers: Can matter be subdivided into fundamental particles? Democritus (460–370 BC): All matter can be divided into indivisible atomos. Dalton: proposed atomic theory with the following postulates: • Elements are composed of atoms. • All atoms of an element are identical. • In chemical ...
Chemistry - School District of Springfield Township
... o The half-life of a radioactive element is the time it takes for one-half of the unstable nuclei in a sample to decay. o This reaction (either through fission or fusion) can convert a small mass into a large amount of energy according to Einstein’s equation: E = mc2. o This radiation has many usefu ...
... o The half-life of a radioactive element is the time it takes for one-half of the unstable nuclei in a sample to decay. o This reaction (either through fission or fusion) can convert a small mass into a large amount of energy according to Einstein’s equation: E = mc2. o This radiation has many usefu ...
Bonding and Nomenclature
... hydrogen bonded to F, O, or N. F, O, and N are very electronegative so it is a very strong dipole. The hydrogen partially share with the lone pair in the molecule next to it. The strongest of the intermolecular forces. ...
... hydrogen bonded to F, O, or N. F, O, and N are very electronegative so it is a very strong dipole. The hydrogen partially share with the lone pair in the molecule next to it. The strongest of the intermolecular forces. ...
AP_PPT_ch_2
... Postulated the existence of negatively charged particles, that we now call electrons, using cathode-ray tubes. Determined the charge-to-mass ratio of an electron. The atom must also contain positive particles that balance exactly the negative charge carried by electrons. ...
... Postulated the existence of negatively charged particles, that we now call electrons, using cathode-ray tubes. Determined the charge-to-mass ratio of an electron. The atom must also contain positive particles that balance exactly the negative charge carried by electrons. ...
Synopses - Mindfiesta
... molecules and ions in terms of the shared pairs of electrons and the octet rule. The lowest energy structure is the one with the smallest formal charges on the atoms. The formal charge is a factor based on a pure covalent view of bonding in which electron pairs are shared equally by neighbouring ato ...
... molecules and ions in terms of the shared pairs of electrons and the octet rule. The lowest energy structure is the one with the smallest formal charges on the atoms. The formal charge is a factor based on a pure covalent view of bonding in which electron pairs are shared equally by neighbouring ato ...
printer-friendly version
... Students know the number of electrons in an atom determines whether the atom is electrically neutral or an ion. E/S All around us are objects. In school, we see desks, chairs, books, students and teachers. Perhaps you ride to and from school on a school bus that drives over a road paved in asphalt, ...
... Students know the number of electrons in an atom determines whether the atom is electrically neutral or an ion. E/S All around us are objects. In school, we see desks, chairs, books, students and teachers. Perhaps you ride to and from school on a school bus that drives over a road paved in asphalt, ...
Basic Chemistry Part 1 Presentation
... nucleus holding up to 2 electrons L or 2nd Shell – can hold up to a maximum of 8 electrons M or 3rd shell – can hold up to a maximum of 18 electrons ...
... nucleus holding up to 2 electrons L or 2nd Shell – can hold up to a maximum of 8 electrons M or 3rd shell – can hold up to a maximum of 18 electrons ...
Atoms and nukes packet 2016
... Indiana Jones and Nicholas Cage are searching for buried treasures again. They think they may find something in your backyard! They’re searching for 30 million year old fish fossils, 2000 year old cups and billion year old rocks! Radioactive dating will help us estimate the age of some objects. But ...
... Indiana Jones and Nicholas Cage are searching for buried treasures again. They think they may find something in your backyard! They’re searching for 30 million year old fish fossils, 2000 year old cups and billion year old rocks! Radioactive dating will help us estimate the age of some objects. But ...
Introductory Chemistry: A Foundation, 6th Ed. Introductory Chemistry
... of any other element. – Carbon atoms have different chemical and physical properties than sulfur atoms. ...
... of any other element. – Carbon atoms have different chemical and physical properties than sulfur atoms. ...
Atoms Worksheet
... Describe the speed atoms move in a solid, liquid and gas each. Recall our information from last week for the “particles” that make up each state of matter. ____________________________________________________________________________________ __________________________________________________________ ...
... Describe the speed atoms move in a solid, liquid and gas each. Recall our information from last week for the “particles” that make up each state of matter. ____________________________________________________________________________________ __________________________________________________________ ...
Electrons
... a. an electron circles the nucleus only in fixed energy ranges called orbits; b. an electron can neither gain or lose energy inside this orbit, but could move up or down to another orbit; c. that the lowest energy orbit is closest to the nucleus. Describe the wave/particle duality of electrons. Writ ...
... a. an electron circles the nucleus only in fixed energy ranges called orbits; b. an electron can neither gain or lose energy inside this orbit, but could move up or down to another orbit; c. that the lowest energy orbit is closest to the nucleus. Describe the wave/particle duality of electrons. Writ ...
Name: Empower Yourself Chemistry Empower Your Community
... 4. Answer questions a and b using the information below and your class notes. In 1897, J.J. Thomson demonstrated in an experiment that cathode rays were deflected by an electric field. This suggested that cathode rays were composed of negatively charged particles found in all atoms. Thomson conclude ...
... 4. Answer questions a and b using the information below and your class notes. In 1897, J.J. Thomson demonstrated in an experiment that cathode rays were deflected by an electric field. This suggested that cathode rays were composed of negatively charged particles found in all atoms. Thomson conclude ...
Chapter 8 Periodic Properties of the Element
... General Trends in First Ionization Energy • The larger the effective nuclear charge on the electron, the more energy it takes to remove it. • The farther the most probable distance the electron is from the nucleus, the less energy it ...
... General Trends in First Ionization Energy • The larger the effective nuclear charge on the electron, the more energy it takes to remove it. • The farther the most probable distance the electron is from the nucleus, the less energy it ...
Joseph Proust: Law of Definite Proportions
... not be possible. If not for the discovery of the nucleus, we could not know about the important parts that it consists of (protons and neutrons). Also, the periodic table of elements that we all know so well (from intense memorization for Chemistry tests), the elements would not be ordered the same ...
... not be possible. If not for the discovery of the nucleus, we could not know about the important parts that it consists of (protons and neutrons). Also, the periodic table of elements that we all know so well (from intense memorization for Chemistry tests), the elements would not be ordered the same ...
chemistry - billpalmer
... atoms 2) All atoms of the same element are identical; different atoms are different 3) Atoms cannot be subdivided, created, or destroyed 4) atoms combine in simple whole number ratios to form chemical compounds 5) In chemical reactions, atoms are combined, separated, or rearranged ...
... atoms 2) All atoms of the same element are identical; different atoms are different 3) Atoms cannot be subdivided, created, or destroyed 4) atoms combine in simple whole number ratios to form chemical compounds 5) In chemical reactions, atoms are combined, separated, or rearranged ...
7-1 Notes: Arranging the Elements
... energy level. More than half of the nonmetals are _________ at room temperature. Metalloids, also called _______________ or semiconductors, are the elements that border the zigzag line. Atoms of metalloids have about ________ a complete set of electrons in their outer energy level. Each square on th ...
... energy level. More than half of the nonmetals are _________ at room temperature. Metalloids, also called _______________ or semiconductors, are the elements that border the zigzag line. Atoms of metalloids have about ________ a complete set of electrons in their outer energy level. Each square on th ...