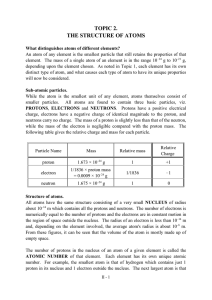
TOPIC 2. THE STRUCTURE OF ATOMS
... electrostatics would require the electrons to collapse into the nucleus due to the attraction of the protons. Because this does not happen, there must be other laws which govern the behaviour of electrons in atoms. Models to explain this will be presented later in the year as part of all first year ...
... electrostatics would require the electrons to collapse into the nucleus due to the attraction of the protons. Because this does not happen, there must be other laws which govern the behaviour of electrons in atoms. Models to explain this will be presented later in the year as part of all first year ...
File
... 6. Matter is anything that has a mass and takes up space. An element is the simplest form of matter, which cannot be broken down any further. Elements are listed on Table S and the periodic table. Their symbols start with an uppercase letter. a. Which of the following is not matter? ________________ ...
... 6. Matter is anything that has a mass and takes up space. An element is the simplest form of matter, which cannot be broken down any further. Elements are listed on Table S and the periodic table. Their symbols start with an uppercase letter. a. Which of the following is not matter? ________________ ...
TOPIC 2. THE STRUCTURE OF ATOMS
... the normal laws of electrostatics would require the electrons to collapse into the nucleus due to the attraction of the protons. Because this does not happen, there must be other laws which govern the behaviour of electrons in atoms. Models to explain this will be presented later in the year as part ...
... the normal laws of electrostatics would require the electrons to collapse into the nucleus due to the attraction of the protons. Because this does not happen, there must be other laws which govern the behaviour of electrons in atoms. Models to explain this will be presented later in the year as part ...
m03_che_sb_ibdip_9755_u03
... the Periodic Table took many years and involved scientists from different countries building upon the foundations of each other’s work and ideas. ...
... the Periodic Table took many years and involved scientists from different countries building upon the foundations of each other’s work and ideas. ...
Bohr, Niels Henrik David
... their atoms and that only the atomic weight and possible radioactive behaviour are determined by the small but massive nucleus itself. Rutherford's nuclear atom was both mechanically and electromagnetically unstable, but Bohr imposed stability on it by introducing the new and not yet clarified idea ...
... their atoms and that only the atomic weight and possible radioactive behaviour are determined by the small but massive nucleus itself. Rutherford's nuclear atom was both mechanically and electromagnetically unstable, but Bohr imposed stability on it by introducing the new and not yet clarified idea ...
Periodic Table ppt
... • The electronegativity of an element indicates its relative ability to * • Electronegativity decreases down a group and increases left to right across a period. ...
... • The electronegativity of an element indicates its relative ability to * • Electronegativity decreases down a group and increases left to right across a period. ...
Chapter 6.2 Notes
... - because they do not form individual molecules, to write the chemical formulas use the smallest ratio of one ion to another, called the formula unit NaCl 1:1 Na2O 2:1 AlBr3 1:3 - smallest ratio means they will not be divisible by each other and get a whole number - will never have an ionic compound ...
... - because they do not form individual molecules, to write the chemical formulas use the smallest ratio of one ion to another, called the formula unit NaCl 1:1 Na2O 2:1 AlBr3 1:3 - smallest ratio means they will not be divisible by each other and get a whole number - will never have an ionic compound ...
TOPIC 2. THE STRUCTURE OF ATOMS
... which has atomic number 10, one smaller than the Na atom. All the other members of the first group of elements in Table 2 (the alkali metals) also have just one more electron than a noble gas atom, and they all behave as does sodium in that relatively little energy is needed to form their +1 cations ...
... which has atomic number 10, one smaller than the Na atom. All the other members of the first group of elements in Table 2 (the alkali metals) also have just one more electron than a noble gas atom, and they all behave as does sodium in that relatively little energy is needed to form their +1 cations ...
The Nuclear Atom
... The net charge is represented by a superscript. Superscripts +, 2+, and 3+ mean a net charge resulting from the loss of one, two, or three electrons. Superscripts -, 2-, and 3- mean a net charge resulting from the gain of one, two, or three electrons. ...
... The net charge is represented by a superscript. Superscripts +, 2+, and 3+ mean a net charge resulting from the loss of one, two, or three electrons. Superscripts -, 2-, and 3- mean a net charge resulting from the gain of one, two, or three electrons. ...
p,d,f
... Learning objective 1.15 The student can justify the selection of a particular type of spectroscopy to measure properties associated with vibrational or electronic motions of molecules. [See SP 4.1, 6.4; Essential knowledge 1.D.3] Learning objective 1.16 The student can design and/or interpret the re ...
... Learning objective 1.15 The student can justify the selection of a particular type of spectroscopy to measure properties associated with vibrational or electronic motions of molecules. [See SP 4.1, 6.4; Essential knowledge 1.D.3] Learning objective 1.16 The student can design and/or interpret the re ...
atomic mass - Belle Vernon Area School District
... • It was discovered that the same element could have atoms with different masses called ...
... • It was discovered that the same element could have atoms with different masses called ...
Introductory Chemistry, 2nd Edition Nivaldo Tro
... • The number of protons in the nucleus of an atom is called the atomic number. Z is the short-hand designation for the atomic number. Because each element’s atoms have a unique number of protons, each element can be identified by its atomic number. The elements are arranged on the Periodic Tab ...
... • The number of protons in the nucleus of an atom is called the atomic number. Z is the short-hand designation for the atomic number. Because each element’s atoms have a unique number of protons, each element can be identified by its atomic number. The elements are arranged on the Periodic Tab ...
Chapter 10 - HCC Learning Web
... The number of molecular orbitals formed is always equal to the number of atomic orbitals combined. A molecular orbital can accommodate up to two electrons. When electrons are added to orbitals of the same energy, the most stable arrangement is predicted by Hund's rule. Low-energy molecular orbitals ...
... The number of molecular orbitals formed is always equal to the number of atomic orbitals combined. A molecular orbital can accommodate up to two electrons. When electrons are added to orbitals of the same energy, the most stable arrangement is predicted by Hund's rule. Low-energy molecular orbitals ...
Name: Per: Date: Unit 1. Materials: Formulating Matter B. Periodic
... properties are placed in the same columns. Physical properties vary in predictable patterns across rows and down columns. ...
... properties are placed in the same columns. Physical properties vary in predictable patterns across rows and down columns. ...
Organizing the periodic table
... The vertical columns of the periodic table are known as a group. Another name for each group is a “family”. Each group is filled with atoms which have similar characteristics. There are eighteen groups in the periodic table. The lanthanides and actinides do not fit in the periodic table because the ...
... The vertical columns of the periodic table are known as a group. Another name for each group is a “family”. Each group is filled with atoms which have similar characteristics. There are eighteen groups in the periodic table. The lanthanides and actinides do not fit in the periodic table because the ...
Periodic Classification of Elements
... 6. This is due to the addition of one extra shell from one element to another. Ionization Energy: 1. The minimum energy required to remove an electron from the outermost orbital of an atom in the gaseous state" is called ionisation energy. 2. Its units are electron volt (or) kilo joules/mole. 3. In ...
... 6. This is due to the addition of one extra shell from one element to another. Ionization Energy: 1. The minimum energy required to remove an electron from the outermost orbital of an atom in the gaseous state" is called ionisation energy. 2. Its units are electron volt (or) kilo joules/mole. 3. In ...
chapter2
... • In the written description, fluorine is said to have 9 protons and 10 neutrons (the mass number is the sum of the numbers of protons and neutrons). • In the symbol, the number 19 in written in the mass number or A (upper left) position. • Note: The periodic table does not show the mass number for ...
... • In the written description, fluorine is said to have 9 protons and 10 neutrons (the mass number is the sum of the numbers of protons and neutrons). • In the symbol, the number 19 in written in the mass number or A (upper left) position. • Note: The periodic table does not show the mass number for ...
Chapter 5 - Valencia College
... indestructible particles called atoms. 1. All atoms of an element are identical and have the same properties. 2. Atoms of different elements combine to form compounds. 3. Compounds contain atoms in small whole number ratios. 4. Atoms can combine in more than one ratio to form different compounds. Ch ...
... indestructible particles called atoms. 1. All atoms of an element are identical and have the same properties. 2. Atoms of different elements combine to form compounds. 3. Compounds contain atoms in small whole number ratios. 4. Atoms can combine in more than one ratio to form different compounds. Ch ...
Usefulness of the periodic table in studying the chemistry of elements:
... In this lesson, we will discuss the classification of elements in the Periodic Table. The Periodic Table helps us in the prediction of the properties of new compounds by comparison of the properties of known compounds. The modern Periodic Table is an arrangement of all the chemical elements in the o ...
... In this lesson, we will discuss the classification of elements in the Periodic Table. The Periodic Table helps us in the prediction of the properties of new compounds by comparison of the properties of known compounds. The modern Periodic Table is an arrangement of all the chemical elements in the o ...
Chapter 2 1
... At both the symbolic and molecular levels, chemists employ “atoms” as the basic building block. Literally, “atoms” means “not cuttable” . It is a term that originates in ancient Greece with a philosopher named “Demokritus of Abdera”. Although you can not “see” atoms in the same sense that you can s ...
... At both the symbolic and molecular levels, chemists employ “atoms” as the basic building block. Literally, “atoms” means “not cuttable” . It is a term that originates in ancient Greece with a philosopher named “Demokritus of Abdera”. Although you can not “see” atoms in the same sense that you can s ...
chapter 2: atoms, ions, and molecules
... compound (eg. NaCl, Al2O3, etc.) – The formula gives the ratio of ions (not actual #). – The 3D representation of NaCl at the right shows a network of Na+ (purple) and Cl– ions (green). – The formula, NaCl, indicates a 1-to-1 ratio of Na+ ions and Cl– ions present, not the presence of only one ion o ...
... compound (eg. NaCl, Al2O3, etc.) – The formula gives the ratio of ions (not actual #). – The 3D representation of NaCl at the right shows a network of Na+ (purple) and Cl– ions (green). – The formula, NaCl, indicates a 1-to-1 ratio of Na+ ions and Cl– ions present, not the presence of only one ion o ...