Document
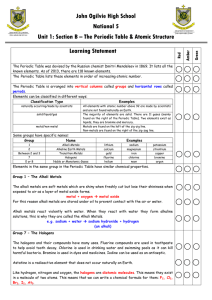
... 29. Tell whether each of the following elements is an inner transition metal, a noble gas, an alkali metal, an alkaline earth metal, or a halogen. Then give its period and group numbers. a. calcium b. cesium c. fluorine d. chromium ...
... 29. Tell whether each of the following elements is an inner transition metal, a noble gas, an alkali metal, an alkaline earth metal, or a halogen. Then give its period and group numbers. a. calcium b. cesium c. fluorine d. chromium ...
Atomic Models 2015-2016
... • To make molecules, you must have elements. • Elements are made of atoms. While the atoms may have different weights and organization, they are all built in the same way. ...
... • To make molecules, you must have elements. • Elements are made of atoms. While the atoms may have different weights and organization, they are all built in the same way. ...
elements_and_the_periodic_table_2011
... Atoms are the smallest part of an element that has all the properties of an element. ...
... Atoms are the smallest part of an element that has all the properties of an element. ...
Name
... • An atom is the smallest particle of an element that retains the chemical properties of that element. • The nucleus is a very small region located at the center of an atom. • The nucleus is made up of at least one positively charged particle called a proton and usually one or more neutral particles ...
... • An atom is the smallest particle of an element that retains the chemical properties of that element. • The nucleus is a very small region located at the center of an atom. • The nucleus is made up of at least one positively charged particle called a proton and usually one or more neutral particles ...
The History of Atomic Theory
... Described the atom as a small, incompressible sphere with an atmosphere of heat. ...
... Described the atom as a small, incompressible sphere with an atmosphere of heat. ...
Periodic Table Jeopardy
... A substance that cannot be separated or broken down into simpler substances by chemical means. All atoms in this substance have the same atomic #. ...
... A substance that cannot be separated or broken down into simpler substances by chemical means. All atoms in this substance have the same atomic #. ...
History of Chemistry PPT
... elements where the middle element has properties that were an average of the other two members when ordered by the atomic weight (Li,Na,K) ...
... elements where the middle element has properties that were an average of the other two members when ordered by the atomic weight (Li,Na,K) ...
CHAPTER 4 EXAM: THE NATURE OF THE ATOM (modified)
... ____ 26. Which form of radiation is the most penetrating? a. alpha c. X-ray b. gamma Short Answer 27. What is the average atomic mass of this element? SHOW ALL WORK!!! Isotope Mass (amu) Percent Abundance Phosphorus-29 ...
... ____ 26. Which form of radiation is the most penetrating? a. alpha c. X-ray b. gamma Short Answer 27. What is the average atomic mass of this element? SHOW ALL WORK!!! Isotope Mass (amu) Percent Abundance Phosphorus-29 ...
Unit 1 Review Sheet
... 2. Explain why it takes more energy to remove a 4s electron from zinc than from calcium. ...
... 2. Explain why it takes more energy to remove a 4s electron from zinc than from calcium. ...
Atomic Size
... As you move down a group in the periodic table, atomic size generally increases. • WHY? ...
... As you move down a group in the periodic table, atomic size generally increases. • WHY? ...
File
... Substances formed when two or more different elements chemically combine—these have properties that differ from the elements that make them up ...
... Substances formed when two or more different elements chemically combine—these have properties that differ from the elements that make them up ...
Anticipation Guide for Chem Talk pg 370-373
... Masses of Elements and Compounds in a Reaction: My Author’s Statement: opinion opinion T or F ...
... Masses of Elements and Compounds in a Reaction: My Author’s Statement: opinion opinion T or F ...
Atoms The smallest piece of matter that have specific properties of
... Found in the atomic nucleus. Neutron (no charge neutrons) No charge (neutral) Found in the nucleus. Electron (negative electrons) Negatively charged particles Found in the outer shells. Electrons determine properties of the atom. Chemical reactions involve sharing or exchanging electrons. ...
... Found in the atomic nucleus. Neutron (no charge neutrons) No charge (neutral) Found in the nucleus. Electron (negative electrons) Negatively charged particles Found in the outer shells. Electrons determine properties of the atom. Chemical reactions involve sharing or exchanging electrons. ...
Atomic Structure Notes Atoms
... Electron cloud or energy rings -Atoms are made of subatomic particles: protons, neutrons, & electrons ...
... Electron cloud or energy rings -Atoms are made of subatomic particles: protons, neutrons, & electrons ...
The Periodic Table - Lincoln Park High School
... of outer-shell electrons result in increased attraction by nucleus for the fewer remaining electrons. • negative ions - anions - always larger than the neutral atom because effective nuclear attraction is less for increased number of electrons ...
... of outer-shell electrons result in increased attraction by nucleus for the fewer remaining electrons. • negative ions - anions - always larger than the neutral atom because effective nuclear attraction is less for increased number of electrons ...
- Elliott Hudson College
... Chemistry Atoms consist of a central ____________ containing protons and ___________. The nucleus is _______ compared to the size of the whole atom. The nucleus is surrounded by ___________ in energy levels (also called _________). Atoms have no electric charge because they contain the same number o ...
... Chemistry Atoms consist of a central ____________ containing protons and ___________. The nucleus is _______ compared to the size of the whole atom. The nucleus is surrounded by ___________ in energy levels (also called _________). Atoms have no electric charge because they contain the same number o ...
atomic
... -negatively charged subatomic particle -found surrounding the nucleus in energy levels or electron clouds -discovered by JJ Thomson in 1897 -mass= 9.11 X 10-28g ...
... -negatively charged subatomic particle -found surrounding the nucleus in energy levels or electron clouds -discovered by JJ Thomson in 1897 -mass= 9.11 X 10-28g ...
Chapter 22- Properties of Atoms and the Periodic Table
... b. Atom- smallest piece of matter that still has the properties of the element i. Protons have electrical charge of 1+. ii. Neutrons do not have an electrical charge. iii. Electrons have electrical charge of 1-. iv. Protons and neutrons are in the nucleus of an atom; electrons surround the nucleus. ...
... b. Atom- smallest piece of matter that still has the properties of the element i. Protons have electrical charge of 1+. ii. Neutrons do not have an electrical charge. iii. Electrons have electrical charge of 1-. iv. Protons and neutrons are in the nucleus of an atom; electrons surround the nucleus. ...
Chapter 10 Power Point - Biloxi Public Schools
... isotopes accounts for the average atomic mass. Average atomic mass - the average mass of the mixture of an element & its isotopes; only a whole number if radioactive or man-made; usually carried to 2 or 3 places past the decimal. The # of protons determines what the element is; the # of neutrons det ...
... isotopes accounts for the average atomic mass. Average atomic mass - the average mass of the mixture of an element & its isotopes; only a whole number if radioactive or man-made; usually carried to 2 or 3 places past the decimal. The # of protons determines what the element is; the # of neutrons det ...
3 Atoms
... o Summarize the five essential points of Dalton’s atomic theory o Explain the relationship between Dalton’s atomic theory and the law of conservation of mass, the law of definite proportions, and the law of multiple proportions o Summarize the experiment of Thompson that led to the discovery of the ...
... o Summarize the five essential points of Dalton’s atomic theory o Explain the relationship between Dalton’s atomic theory and the law of conservation of mass, the law of definite proportions, and the law of multiple proportions o Summarize the experiment of Thompson that led to the discovery of the ...
SB Vocab list Word document
... A negatively charged particle found in the shells of an atom. Its mass is approximately 1/2000 that of a proton or neutron. Group A vertical column in the Periodic Table. Elements in the same group have similar chemical properties since they all have the same number of electrons in their outer (or v ...
... A negatively charged particle found in the shells of an atom. Its mass is approximately 1/2000 that of a proton or neutron. Group A vertical column in the Periodic Table. Elements in the same group have similar chemical properties since they all have the same number of electrons in their outer (or v ...
Name Date Class Period ______
... Name ______________________________________ Date __________________ Class Period _________ Atoms, Elements, and Compound Test Study Guide I. ...
... Name ______________________________________ Date __________________ Class Period _________ Atoms, Elements, and Compound Test Study Guide I. ...
Name: Per: _____ Date: ______ Unit 5 Redemption Packet: The
... questions. If you missed any notes, you will find them at www.starkchemistry.weebly.com. 1. Mendeleev arranged the elements in his periodic table by increasing _______________ ________________. 2. Why did Mendeleev leave spaces in his periodic table? _________________________________________________ ...
... questions. If you missed any notes, you will find them at www.starkchemistry.weebly.com. 1. Mendeleev arranged the elements in his periodic table by increasing _______________ ________________. 2. Why did Mendeleev leave spaces in his periodic table? _________________________________________________ ...