Water - School
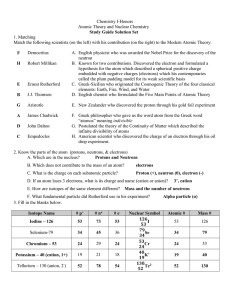
... The number of protons, neutrons and electrons in an atom can be worked out using the atomic number and mass number. Number of protons = ……………………………………………………………………..…………………………………………… Number of neutrons = ………………………………………………………………………………………………………………… Number of electrons = ………………………………………………………………………………… ...
... The number of protons, neutrons and electrons in an atom can be worked out using the atomic number and mass number. Number of protons = ……………………………………………………………………..…………………………………………… Number of neutrons = ………………………………………………………………………………………………………………… Number of electrons = ………………………………………………………………………………… ...
PERIODIC TABLE quiz study guide
... 3. What are the 4 forces that govern atomic behavior (list from strongest to weakest). ...
... 3. What are the 4 forces that govern atomic behavior (list from strongest to weakest). ...
Worksheet 1, UNIT THREE
... 2. This happens because as you move down the group each element has one more occupied __________________________________________ than the last one. 3. As you move from left to right across a period on the periodic table the size of an atom will __________________________________________. 4. This hap ...
... 2. This happens because as you move down the group each element has one more occupied __________________________________________ than the last one. 3. As you move from left to right across a period on the periodic table the size of an atom will __________________________________________. 4. This hap ...
The Periodic Table of Elements
... • Arranged to make predicting the properties of the elements easier. • The order is based on the # of protons an atom of that element has in its nucleus. – This order is known as the periodic law (arranged this way, similarities in their properties will occur in a regular pattern). ...
... • Arranged to make predicting the properties of the elements easier. • The order is based on the # of protons an atom of that element has in its nucleus. – This order is known as the periodic law (arranged this way, similarities in their properties will occur in a regular pattern). ...
Two valence electrons.
... elements by increasing atomic mass, leaving blank spaces where he was sure elements Dmitri yet to be discovered Mendeleev would fit. ...
... elements by increasing atomic mass, leaving blank spaces where he was sure elements Dmitri yet to be discovered Mendeleev would fit. ...
Atoms and the Periodic Table Test
... a. Speed in mph b. stopping distance c. same car, same road conditions, same driver 2) An experiment is run to see if a new type of salt will melt ice better then sodium chloride. The salt is sprinkled on 30 sheets of ice and the time it takes for the ice to melt is recorded. a. What is the experime ...
... a. Speed in mph b. stopping distance c. same car, same road conditions, same driver 2) An experiment is run to see if a new type of salt will melt ice better then sodium chloride. The salt is sprinkled on 30 sheets of ice and the time it takes for the ice to melt is recorded. a. What is the experime ...
Another look at chemical reactions HYDROGEN PEROXIDE WATER
... Atomic terms - ATOMIC NUMBER: The number of protons in the atomic nucleus. Each ELEMENT has the SAME NUMBER OF PROTONS in every nucleus. In neutral atoms, the number of ELECTRONS is also equal to the atomic number. Example: Helium has an atomic number of 2. Every helium atom has two protons in its ...
... Atomic terms - ATOMIC NUMBER: The number of protons in the atomic nucleus. Each ELEMENT has the SAME NUMBER OF PROTONS in every nucleus. In neutral atoms, the number of ELECTRONS is also equal to the atomic number. Example: Helium has an atomic number of 2. Every helium atom has two protons in its ...
Study Guide - Honors Chemistry
... one nucleus is broken into multiple (2 in this case) nuclei by force (an alpha particle is used to break it up) one nucleus is broken into multiple (2 in this case) nuclei on its own. No force is needed. one nucleus is transformed into another nucleus by bombarding a particle into it. A particle may ...
... one nucleus is broken into multiple (2 in this case) nuclei by force (an alpha particle is used to break it up) one nucleus is broken into multiple (2 in this case) nuclei on its own. No force is needed. one nucleus is transformed into another nucleus by bombarding a particle into it. A particle may ...
CHAPTER 18 NOTES
... • J. J. Thomson – an atom contained small, negatively charged particles • Rutherford – proposed that almost all the mass of an atom and all of its positive charges were concentrated in a central atomic nucleus surrounded by electrons ...
... • J. J. Thomson – an atom contained small, negatively charged particles • Rutherford – proposed that almost all the mass of an atom and all of its positive charges were concentrated in a central atomic nucleus surrounded by electrons ...
Atomic Structure
... Early Atomic Theory “Cosmic substance is made up of an infinite number of elements or particles ...
... Early Atomic Theory “Cosmic substance is made up of an infinite number of elements or particles ...
Name - Aurora City Schools
... B__ the first column on the periodic table is also known as this; they are ...
... B__ the first column on the periodic table is also known as this; they are ...
atomic structure map
... Chemistry I Unit 3 Periodic Table and Atomic Structure Dates : 15 days Essential Questions 1. What is an atom? 2. What does an atom look like? 3. What is electromagnetic radiation? 4. How is the Periodic table arranged? Content/Skills: elements, dalton’s atomic theory, structure of the atom, modern ...
... Chemistry I Unit 3 Periodic Table and Atomic Structure Dates : 15 days Essential Questions 1. What is an atom? 2. What does an atom look like? 3. What is electromagnetic radiation? 4. How is the Periodic table arranged? Content/Skills: elements, dalton’s atomic theory, structure of the atom, modern ...
Democritus - The Laughing Philosopher
... which are indivisible and indestructible. 2) All atoms of an element are identical in mass and properties. 3) Compounds are formed by two or more different kinds of atoms coming together. 4) A chemical reaction is a rearrangement of atoms. ...
... which are indivisible and indestructible. 2) All atoms of an element are identical in mass and properties. 3) Compounds are formed by two or more different kinds of atoms coming together. 4) A chemical reaction is a rearrangement of atoms. ...
ATOMIC SIZE
... These are very easy to understand. As we move down the periodic table within a group (same number of electrons in the outer shell), each row represents addition of a new shell of electrons. Those electrons take up space (mostly because they repel one-another), so more shells means a larger atom. The ...
... These are very easy to understand. As we move down the periodic table within a group (same number of electrons in the outer shell), each row represents addition of a new shell of electrons. Those electrons take up space (mostly because they repel one-another), so more shells means a larger atom. The ...
Atomic theory notes
... Atomic Theory John Dalton: Early 1800s ~ discovered 4 parts of atomic theory 1. All elements are composed of atoms that cannot be divided 2. All atoms of the same element are exactly alike and have the same mass 3. An atom of one element cannot be changed into an atom of a different element. Atoms c ...
... Atomic Theory John Dalton: Early 1800s ~ discovered 4 parts of atomic theory 1. All elements are composed of atoms that cannot be divided 2. All atoms of the same element are exactly alike and have the same mass 3. An atom of one element cannot be changed into an atom of a different element. Atoms c ...
Name________________________ Period____ Date
... Name________________________ Period____ Date__________ Chemistry Review Sheet #2 ...
... Name________________________ Period____ Date__________ Chemistry Review Sheet #2 ...
Chapter 14 Inside the Atom Notes
... 1. When a proton is released, one element changes into another, a process called transmutation. ...
... 1. When a proton is released, one element changes into another, a process called transmutation. ...
Atomic theory
... different properties, including mass and chemical reactivity. 4. Atoms are not changed by chemical reactions, but merely rearranged into different compounds. ...
... different properties, including mass and chemical reactivity. 4. Atoms are not changed by chemical reactions, but merely rearranged into different compounds. ...
The Periodic Law
... Arranged his periodic table according to atomic mass so that elements with similar properties were in the same group Predicted the properties of elements that had not yet been discovered using his periodic table ...
... Arranged his periodic table according to atomic mass so that elements with similar properties were in the same group Predicted the properties of elements that had not yet been discovered using his periodic table ...