PowerPoint - Models of the Atom - A Historical Perspective
... Q- Sometimes an isotope is written without its atomic number - e.g. 35S (or S-35). Why? Q- Draw B-R diagrams for the two Li isotopes. A- The atomic # of an element doesn’t change Although the number of neutrons can vary, atoms have definite numbers of protons. ...
... Q- Sometimes an isotope is written without its atomic number - e.g. 35S (or S-35). Why? Q- Draw B-R diagrams for the two Li isotopes. A- The atomic # of an element doesn’t change Although the number of neutrons can vary, atoms have definite numbers of protons. ...
Atomic Model Timeline
... Five parts to theory Also includes element’s atoms are identical in mass Described nature of cathode rays Plum pudding model of the atom Discovered electron Measured charge of an electron Oil drop experiment Completed with Thomson Atoms can emit “quanta” Einstein used this scientis ...
... Five parts to theory Also includes element’s atoms are identical in mass Described nature of cathode rays Plum pudding model of the atom Discovered electron Measured charge of an electron Oil drop experiment Completed with Thomson Atoms can emit “quanta” Einstein used this scientis ...
Concepts of Physical Science
... 4. An element that is nonmalleable, nonductile, and a poor conductor of electricity and heat 5. the core of an atom, consisting of two basic subatomic particles- protons and neutrons 6. A substance with a pH more than 7 that turns litmus paper blue 7. a chemical bond in which atoms are held together ...
... 4. An element that is nonmalleable, nonductile, and a poor conductor of electricity and heat 5. the core of an atom, consisting of two basic subatomic particles- protons and neutrons 6. A substance with a pH more than 7 that turns litmus paper blue 7. a chemical bond in which atoms are held together ...
Pre-Test Atomic Structure
... 5. An atom is usually neutrally charged. This means the number of protons must equal the number of ______________. 6. The atomic number of an atom equals the number of ____________ 7. All atoms of the same element will have the same number of ________________ 8. The periodic table is arranged in ord ...
... 5. An atom is usually neutrally charged. This means the number of protons must equal the number of ______________. 6. The atomic number of an atom equals the number of ____________ 7. All atoms of the same element will have the same number of ________________ 8. The periodic table is arranged in ord ...
Chapter 3 notes
... • Orbital- or energy shell/level is a region in an atom where there is a high probability of finding electrons. • Valence electron- an electron in the outermost energy level of an atom. So in Al valence 3. ...
... • Orbital- or energy shell/level is a region in an atom where there is a high probability of finding electrons. • Valence electron- an electron in the outermost energy level of an atom. So in Al valence 3. ...
File - Mrs. Riggs Online
... losing electrons will cause an atom’s charge to be unbalanced: -ion: atom that has an electrical charge because of losing or gaining electrons -anions are (-) ions -cations are (+) ions ● Mass number: total number of protons + ne ...
... losing electrons will cause an atom’s charge to be unbalanced: -ion: atom that has an electrical charge because of losing or gaining electrons -anions are (-) ions -cations are (+) ions ● Mass number: total number of protons + ne ...
Chapter 6 Periodic law- states that when the elements are arranged
... Nonmetal- Elements that are generally gases or dull, brittle solids that are poor conductors of heat and electricity Halogen- A highly reactive group 7A element Noble gas- An extremely uncreative group 8A element Metalloid- An element, such a s silicon or germanium, that has physical and chemical pr ...
... Nonmetal- Elements that are generally gases or dull, brittle solids that are poor conductors of heat and electricity Halogen- A highly reactive group 7A element Noble gas- An extremely uncreative group 8A element Metalloid- An element, such a s silicon or germanium, that has physical and chemical pr ...
The Atomic Model - Mr. Brown`s Science Town
... positive dense core of the atom containing protons and neutrons Atom is mostly empty space where electrons randomly orbit nucleus. ...
... positive dense core of the atom containing protons and neutrons Atom is mostly empty space where electrons randomly orbit nucleus. ...
Chapter 3 - CCRI Faculty Web
... •Mass Number – The number of protons + the number of neutrons in an atom. For example: An atom with 5 protons and 7 neutrons •Mass # = 5p+ + 7n0 = 12 •This number is not unique for each element. All but one element have atoms with different numbers of neutrons and therefore different mass numbers. ...
... •Mass Number – The number of protons + the number of neutrons in an atom. For example: An atom with 5 protons and 7 neutrons •Mass # = 5p+ + 7n0 = 12 •This number is not unique for each element. All but one element have atoms with different numbers of neutrons and therefore different mass numbers. ...
Chap 11 Sect 1 Notes Atomic Theory
... •Electrons were free to rotate within the cloud of positive substance. ...
... •Electrons were free to rotate within the cloud of positive substance. ...
Honors review- ch. 4 Element Symbol Atomic # Atomic mass
... 1. 65.38 is the average of all the different isotopes of zinc. Protons and neutrons each have a relative mass of 1 amu, so the average mass should not be a fraction of a number. 2. If two atoms have the same number of protons then they are the same element. Atoms of the same element with a different ...
... 1. 65.38 is the average of all the different isotopes of zinc. Protons and neutrons each have a relative mass of 1 amu, so the average mass should not be a fraction of a number. 2. If two atoms have the same number of protons then they are the same element. Atoms of the same element with a different ...
The Atom - Mrs. Ellis` Science Class!
... o These were just ideas, not truly science _________________(next 2000 years) o Mixture of science and mysticism o Lab procedures were developed, but did not perform control experiments like real scientists ____________________________ o __________________________Model Atom is a small ________ ...
... o These were just ideas, not truly science _________________(next 2000 years) o Mixture of science and mysticism o Lab procedures were developed, but did not perform control experiments like real scientists ____________________________ o __________________________Model Atom is a small ________ ...
Chapter 4 Study Guide
... Bohr’s Model is known as the __________________________ Model What is the purpose of energy levels in relation to an electron? ...
... Bohr’s Model is known as the __________________________ Model What is the purpose of energy levels in relation to an electron? ...
Chem Unit 2 Review Guide ANSWERS
... Conservation of Mass apply to each type of reaction? Chemical reactions only involve the atoms’ valence electrons. In a nuclear reaction, the nucleus is actually altered. The Law of Conservation of Mass holds true during chemical reactions, but is not during a nuclear reaction, as mass is converted ...
... Conservation of Mass apply to each type of reaction? Chemical reactions only involve the atoms’ valence electrons. In a nuclear reaction, the nucleus is actually altered. The Law of Conservation of Mass holds true during chemical reactions, but is not during a nuclear reaction, as mass is converted ...
Atomic Structure Atoms
... Atomic Structure The nature of atoms was deduced from important experiments conducted in the late 1800's up to the mid 1900's by scientists in Europe. Some of these include: Wilhelm Röntgen's discovery of x-rays in 1895. Antoine-Henri Becquerel's discovery of radioactivity one year later. J. J. Thom ...
... Atomic Structure The nature of atoms was deduced from important experiments conducted in the late 1800's up to the mid 1900's by scientists in Europe. Some of these include: Wilhelm Röntgen's discovery of x-rays in 1895. Antoine-Henri Becquerel's discovery of radioactivity one year later. J. J. Thom ...
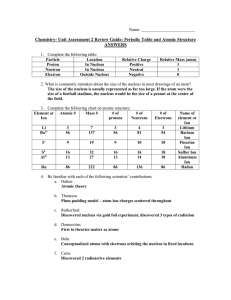
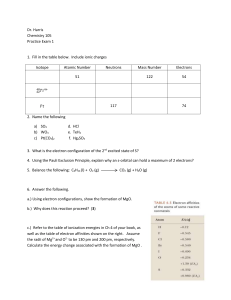
Dr. Harris Chemistry 105 Practice Exam 1 Isotope Atomic Number
... the radii of Mg2+ and O2- to be 130 pm and 200 pm, respectively. Calculate the energy change associated with the formation of MgO . ...
... the radii of Mg2+ and O2- to be 130 pm and 200 pm, respectively. Calculate the energy change associated with the formation of MgO . ...
CLASSIFICATION OF THE ELEMENTS
... ________ 12. Removing one electron from an atom results in the formation of a positive ion with a 11 charge. ________ 13. The relative radii of atoms are estimated as being half the distance between nuclei in diatomic molecules. ________ 14. Atoms with high electronegativity tend to form positive io ...
... ________ 12. Removing one electron from an atom results in the formation of a positive ion with a 11 charge. ________ 13. The relative radii of atoms are estimated as being half the distance between nuclei in diatomic molecules. ________ 14. Atoms with high electronegativity tend to form positive io ...
Atomic Structure
... • Democritus (460 – 370 BCE) • Among first to suggest existence of atoms • Believed atoms were indivisible and indestructible ...
... • Democritus (460 – 370 BCE) • Among first to suggest existence of atoms • Believed atoms were indivisible and indestructible ...
Homework 1B1 - 3 - Uddingston Grammar School
... 7. Sodium has 10 electrons. (a) Complete the diagram to show how the electrons are arranged. You may wish to use the data booklet to help you. ...
... 7. Sodium has 10 electrons. (a) Complete the diagram to show how the electrons are arranged. You may wish to use the data booklet to help you. ...
How to write up a practical: General review
... electrons and neutrons and some of their properties TO BE ABLE draw the basic structure of the atom. TO UNDERSTAND how these particles are physically arranged in relation to each other. ...
... electrons and neutrons and some of their properties TO BE ABLE draw the basic structure of the atom. TO UNDERSTAND how these particles are physically arranged in relation to each other. ...