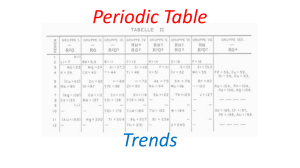
Mid-Term OR Study Guide
... Be able to complete an AUFBAU CHART: Consider the element barium. Answer the following questions: (A) What is the element symbol? How many electrons are in a neutral atom of barium? (B) Draw the orbital notation for barium using up and down arrows. (C) Write the complete electron configuration for b ...
... Be able to complete an AUFBAU CHART: Consider the element barium. Answer the following questions: (A) What is the element symbol? How many electrons are in a neutral atom of barium? (B) Draw the orbital notation for barium using up and down arrows. (C) Write the complete electron configuration for b ...
10/3/16 Intro Atomic Theories
... atomic mass of 64. What is its atomic number? How many electrons and neutrons does it have? ...
... atomic mass of 64. What is its atomic number? How many electrons and neutrons does it have? ...
The Periodic Table
... S-block elements- valence electrons are filling the s orbitals- Groups 1-2 D-block elements-valence electrons are filling the d orbtials- Groups 3-12 P-block elements-valence electrons are filling the p orbitals- Groups 13-18 except He F-block elements-valence electrons are filling the f orbitals- L ...
... S-block elements- valence electrons are filling the s orbitals- Groups 1-2 D-block elements-valence electrons are filling the d orbtials- Groups 3-12 P-block elements-valence electrons are filling the p orbitals- Groups 13-18 except He F-block elements-valence electrons are filling the f orbitals- L ...
Learning Targets Chapter 4
... relative mass of protons (p+) , neutrons (n0) and electrons (e-) in an atom. I can calculate the number of protons (p+) , neutrons (n0) and electrons (e-) in an atom using the atomic number, mass number and overall charge of the atom or a periodic table provided. I can describe the similarity and di ...
... relative mass of protons (p+) , neutrons (n0) and electrons (e-) in an atom. I can calculate the number of protons (p+) , neutrons (n0) and electrons (e-) in an atom using the atomic number, mass number and overall charge of the atom or a periodic table provided. I can describe the similarity and di ...
File
... 32. Give an example of a compound. H2O 33. What is a molecule? An element with more than one atom attached to it 34. Give an example of a molecule. O₂- air we breathe O₃- ozone layer 35. As you go from left to right on the periodic table, describe the changes that occur to element's atomic structure ...
... 32. Give an example of a compound. H2O 33. What is a molecule? An element with more than one atom attached to it 34. Give an example of a molecule. O₂- air we breathe O₃- ozone layer 35. As you go from left to right on the periodic table, describe the changes that occur to element's atomic structure ...
The Periodic Table
... then the configuration will be: 1s22s22p6 • sodium will have a full octet ...
... then the configuration will be: 1s22s22p6 • sodium will have a full octet ...
Elements, Compounds, and Atoms Video Notes
... 8. Ernest Rutherford’s Model of the Atom – developed a more complete understanding of the structure of atoms. He used gold foil and gold atoms. He discovered that atoms are mostly made of ...
... 8. Ernest Rutherford’s Model of the Atom – developed a more complete understanding of the structure of atoms. He used gold foil and gold atoms. He discovered that atoms are mostly made of ...
Unit 4 Review Sheet
... b. Contains 7 electrons in the 3d level d. Has 3 electrons in the 2p orbital 7. What group of elements has completely filled outer energy levels? 8. Which subatomic particle plays the greatest role in determining the physical and chemical properties of an element? ...
... b. Contains 7 electrons in the 3d level d. Has 3 electrons in the 2p orbital 7. What group of elements has completely filled outer energy levels? 8. Which subatomic particle plays the greatest role in determining the physical and chemical properties of an element? ...
What are Elements
... Lavoisier was a pioneer in the field of alchemy. He defined elements as pure substances that cannot be decomposed into simpler substances by means of a chemical change. His careful measurement of mass, allowed him to make accurate inferences about what was happening to the substances. The law of Def ...
... Lavoisier was a pioneer in the field of alchemy. He defined elements as pure substances that cannot be decomposed into simpler substances by means of a chemical change. His careful measurement of mass, allowed him to make accurate inferences about what was happening to the substances. The law of Def ...
Periodic Table Vocabulary Periodic Table – a chart that organizes
... – an element that has some properties of a metal and some properties of a nonmetal. The metalloids are found on the boron staircase, there are 7 metalloids: B, Si, Ge, As, Sb, Te, and Po. ...
... – an element that has some properties of a metal and some properties of a nonmetal. The metalloids are found on the boron staircase, there are 7 metalloids: B, Si, Ge, As, Sb, Te, and Po. ...
Chemical-Periodicity
... down a group. • Atomic size generally decreases as you move from left to right across a period. Largest atoms are towards the bottom and to the left of the periodic table. ...
... down a group. • Atomic size generally decreases as you move from left to right across a period. Largest atoms are towards the bottom and to the left of the periodic table. ...
Diapositiva 1
... – Some particles were deflected at large angles – Discovery: atoms have a very dense positive (+) center, called the nucleus ...
... – Some particles were deflected at large angles – Discovery: atoms have a very dense positive (+) center, called the nucleus ...
Practice Test #2 - smhs
... for Ag as 107.868 amu. Find the percent abundance of the lighter isotope of Ag. 107.868 = 106.9041 (X) + 108.9047 (1.000 - X) -1.0367 = -2.0006 X X = 0.51819 ...
... for Ag as 107.868 amu. Find the percent abundance of the lighter isotope of Ag. 107.868 = 106.9041 (X) + 108.9047 (1.000 - X) -1.0367 = -2.0006 X X = 0.51819 ...
Name - wsscience
... History has recorded many thoughtful leaders, their views and supportive research to unlock the keys of atomic structure. Greek philosopher _______________________ suggested that materials are made of small ______________________ units he called “atomos” leading to the modern name of _______________ ...
... History has recorded many thoughtful leaders, their views and supportive research to unlock the keys of atomic structure. Greek philosopher _______________________ suggested that materials are made of small ______________________ units he called “atomos” leading to the modern name of _______________ ...
periodic table quiz review
... In a neutral atom, the number of protons matches the number of _________________ . The negative particles of an atom are _________________ . The positive particles of an atom are _________________ . The neutral particles of an atom are _________________ . The _________________ and _________________ ...
... In a neutral atom, the number of protons matches the number of _________________ . The negative particles of an atom are _________________ . The positive particles of an atom are _________________ . The neutral particles of an atom are _________________ . The _________________ and _________________ ...
Atomic Structure and Periodic Table Worksheet
... Anything that takes up space is called ___________________ . There are three states of matter; ___________________, ___________________ and ___________________. For example, water exists in ___________________ form as rivers, lakes and streams. Water in a solid form is called ___________________. Wh ...
... Anything that takes up space is called ___________________ . There are three states of matter; ___________________, ___________________ and ___________________. For example, water exists in ___________________ form as rivers, lakes and streams. Water in a solid form is called ___________________. Wh ...
Atomic Structure 1. Historical perspective of the model of the atom a
... of the same type of element have the same chemical properties, compounds are formed by two or more different types of atoms, and that a chemical reaction involves either, joining, separating, or rearranging atoms. b.) In 1910, Ernest Rutherford passed alpha particles through gold foil which showed t ...
... of the same type of element have the same chemical properties, compounds are formed by two or more different types of atoms, and that a chemical reaction involves either, joining, separating, or rearranging atoms. b.) In 1910, Ernest Rutherford passed alpha particles through gold foil which showed t ...
Chemistry I Honors – Semester Exam Review – Fall 2000
... Matter – Ch. 1 6. Classify the following substances as solid, liquid, gas, or plasma based on their properties. a. flexible volume, high KE, particles can disperse freely. b. flexible volume, very high KE, particles are charged. c. fixed volume, very low KE, orderly particles. d. fixed volume, low K ...
... Matter – Ch. 1 6. Classify the following substances as solid, liquid, gas, or plasma based on their properties. a. flexible volume, high KE, particles can disperse freely. b. flexible volume, very high KE, particles are charged. c. fixed volume, very low KE, orderly particles. d. fixed volume, low K ...
Models of the Atom: A Historical perspective
... Aristotle was wrong. However, his theory persisted for 2000 years. ...
... Aristotle was wrong. However, his theory persisted for 2000 years. ...
Goal 4.01
... Given the notation of an element you should be able to determine the number of p, n, and e. The first step is to find the element on the periodic table and determine its atomic number which gives you the number of p. The number of p’s will never change. From there you must determine the number of n ...
... Given the notation of an element you should be able to determine the number of p, n, and e. The first step is to find the element on the periodic table and determine its atomic number which gives you the number of p. The number of p’s will never change. From there you must determine the number of n ...
Biol 1441
... Matter is anything that takes up space and has mass. Element is a substance that cannot be broken down chemically to other substances by chemical reactions. A compound is a substance consisting of two or more different elements combined in a fixed ratio. Essential Elements of Life: Carbon, oxygen, h ...
... Matter is anything that takes up space and has mass. Element is a substance that cannot be broken down chemically to other substances by chemical reactions. A compound is a substance consisting of two or more different elements combined in a fixed ratio. Essential Elements of Life: Carbon, oxygen, h ...
Biochemistry I (CHE 418 / 5418)
... Bohr Model of the Atom • Protons and neutrons are located in the nucleus. • Electrons orbit the nucleus in specific orbits with each orbit corresponding to a different energy level. – Ground state (most stable state) when electrons are in energy levels as near as possible to the nucleus – Excited s ...
... Bohr Model of the Atom • Protons and neutrons are located in the nucleus. • Electrons orbit the nucleus in specific orbits with each orbit corresponding to a different energy level. – Ground state (most stable state) when electrons are in energy levels as near as possible to the nucleus – Excited s ...