Chapter 3, Section 1 Inside an Atom
... number of protons. The atomic number is equal to the number of protons in that element. The atomic number is a unique identifying characteristic. ...
... number of protons. The atomic number is equal to the number of protons in that element. The atomic number is a unique identifying characteristic. ...
Periodic Trends Student
... • Decreases as you go to the right and increases as you go down the periodic table. • Going down in a group makes sense. You’re adding an entire energy level. • When moving to the right, you are adding electrons and protons, which increases the charge. The electrons are pulled closer to the nucleus, ...
... • Decreases as you go to the right and increases as you go down the periodic table. • Going down in a group makes sense. You’re adding an entire energy level. • When moving to the right, you are adding electrons and protons, which increases the charge. The electrons are pulled closer to the nucleus, ...
CP CHEMISTRY STUDY GUIDE
... Periodic Table, and explain the difference in the oxidation numbers of metals and nonmetals in terms of atomic structure and position on the Periodic Table. ...
... Periodic Table, and explain the difference in the oxidation numbers of metals and nonmetals in terms of atomic structure and position on the Periodic Table. ...
Periods
... masses increase as you move from the left to the right in a period All atoms of the elements in the same period have the same number of orbitals/levels All atoms of the elements in a specific period have that respective number of ...
... masses increase as you move from the left to the right in a period All atoms of the elements in the same period have the same number of orbitals/levels All atoms of the elements in a specific period have that respective number of ...
Blue File
... Bohr developed a model from the findings of their research which is the widely recognised model that we use today….. With each atom being made up of a number of sub – atomic particles : Protons and Positive (+ ve) Elctrons are Negative (- ve) and Neutrons are Neutral Each element in the Periodic Tab ...
... Bohr developed a model from the findings of their research which is the widely recognised model that we use today….. With each atom being made up of a number of sub – atomic particles : Protons and Positive (+ ve) Elctrons are Negative (- ve) and Neutrons are Neutral Each element in the Periodic Tab ...
L2: The Atom, Standard Notation and Borh
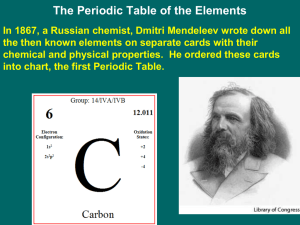
... Use the periodic table (p.647 in your textbook) to help you. The atomic number is on the top left side of each element symbol on the periodic table and the atomic mass is below each element symbol. For example carbon has atomic number 6 and mass of 12. (Note: The mass is rounded to the nearest whole ...
... Use the periodic table (p.647 in your textbook) to help you. The atomic number is on the top left side of each element symbol on the periodic table and the atomic mass is below each element symbol. For example carbon has atomic number 6 and mass of 12. (Note: The mass is rounded to the nearest whole ...
Notes # ____ - The Periodic Table
... Periods are read from left to right and the atomic number increases by one for each element. One way to remember this is that a “period” is often found at the end of a sentence, and sentences are read left right. The period number refers to the number of electron shells the atom has. For example, ...
... Periods are read from left to right and the atomic number increases by one for each element. One way to remember this is that a “period” is often found at the end of a sentence, and sentences are read left right. The period number refers to the number of electron shells the atom has. For example, ...
The Atom - Taylorsville
... • Helped moved alchemy to be chemistry • Mass cannot be created nor destroyed in normal chemical or physical processes. ...
... • Helped moved alchemy to be chemistry • Mass cannot be created nor destroyed in normal chemical or physical processes. ...
Chapter 4
... place in which e-’s are likely to be found ~location depends upon how much nrg the e- has ...
... place in which e-’s are likely to be found ~location depends upon how much nrg the e- has ...
Elements and Atoms
... • To make molecules, you must have elements. • Elements are made of atoms. While the atoms may have different weights and organization, they are all built in the same way. ...
... • To make molecules, you must have elements. • Elements are made of atoms. While the atoms may have different weights and organization, they are all built in the same way. ...
Elements02
... In 1781 Antoine Lavoisier explained how to tell elements from non-elements and over the next 30 years many new elements were discovered by scientists such as Englishman John Dalton. Dalton is also famous for his 1808 theory, which explained why elements differ from each other and non-elements. His t ...
... In 1781 Antoine Lavoisier explained how to tell elements from non-elements and over the next 30 years many new elements were discovered by scientists such as Englishman John Dalton. Dalton is also famous for his 1808 theory, which explained why elements differ from each other and non-elements. His t ...
Lesson Outline - WordPress.com
... * The atoms of one element Cannot be converted into atoms of another element in a chemical reaction. * All atoms of one element have the same properties, such has mass and size * Atoms of different elements combine in specific proportions to form compounds ...
... * The atoms of one element Cannot be converted into atoms of another element in a chemical reaction. * All atoms of one element have the same properties, such has mass and size * Atoms of different elements combine in specific proportions to form compounds ...
Define:
... 43. Express the sum of 8.67 m and 5.2 m to the correct number of significant figures. 44. Express the product of 5.5 mm and 2.00 mm to the correct number of significant figures. 45. List the metric prefixes and their decimal equivalents. Ex: centi .01 46. Make the following conversions: a. 8961 m to ...
... 43. Express the sum of 8.67 m and 5.2 m to the correct number of significant figures. 44. Express the product of 5.5 mm and 2.00 mm to the correct number of significant figures. 45. List the metric prefixes and their decimal equivalents. Ex: centi .01 46. Make the following conversions: a. 8961 m to ...
Name: Date: Period: Page # Evolution of Atomic Theory (Changed
... Atomic Theory Ancient Greeks were the first to think about the nature of _____________ Democritus proposed the idea of matter as small ____________ _________: the smallest particle of an element As more evidence was collected over time, the theory and models were ________ ...
... Atomic Theory Ancient Greeks were the first to think about the nature of _____________ Democritus proposed the idea of matter as small ____________ _________: the smallest particle of an element As more evidence was collected over time, the theory and models were ________ ...
The periodic table
... element is given a 1 or 2 letter abbreviation. The first letter has to be capitalized. If there is a second letter, it is always lowercase. ...
... element is given a 1 or 2 letter abbreviation. The first letter has to be capitalized. If there is a second letter, it is always lowercase. ...
Atoms pg. 102
... Ancient Greeks proposed the idea of atoms. The theory of atoms grew as more evidence was collected over time. ...
... Ancient Greeks proposed the idea of atoms. The theory of atoms grew as more evidence was collected over time. ...
5.1 Structure of the Periodic Table
... The atomic number of an atom is the number of protons in each atom of that element. Atomic Number = Proton Number Because atoms are electrically neutral, the atomic number is also the number of electrons. Atomic Number = Electron Number The mass number tells you the number of protons and neutrons in ...
... The atomic number of an atom is the number of protons in each atom of that element. Atomic Number = Proton Number Because atoms are electrically neutral, the atomic number is also the number of electrons. Atomic Number = Electron Number The mass number tells you the number of protons and neutrons in ...
2.2 Periodic Chart
... The noble gases are non-reactive and stable by themselves. They are all colourless and odourless gases. They glow with distinctive colours (Ne is reddish) when electricity is passed through them. Their ion charges of zero indicate that they do not form charged ions. ...
... The noble gases are non-reactive and stable by themselves. They are all colourless and odourless gases. They glow with distinctive colours (Ne is reddish) when electricity is passed through them. Their ion charges of zero indicate that they do not form charged ions. ...
Chemistry and elements 1. The rows of the periodic table are called
... Chemistry and elements 1. The rows of the periodic table are called: a. classes b. periods c. groups d. families 2. As you move from left to right across the periodic table: a. atomic number decreases b. atomic number increases c. The elements become darker in color d. The elements become lighter in ...
... Chemistry and elements 1. The rows of the periodic table are called: a. classes b. periods c. groups d. families 2. As you move from left to right across the periodic table: a. atomic number decreases b. atomic number increases c. The elements become darker in color d. The elements become lighter in ...
Sept. 28th powerpoint
... Left to right across a period, atomic radius usually decreases. This occurs because each successive element has an added proton and electron which causes the electron to be drawn closer to the nucleus. This decrease in atomic radius also causes the ionization energy to increase when moving from left ...
... Left to right across a period, atomic radius usually decreases. This occurs because each successive element has an added proton and electron which causes the electron to be drawn closer to the nucleus. This decrease in atomic radius also causes the ionization energy to increase when moving from left ...
Atoms, Bonding, and the Periodic Table Understanding Main Ideas
... If the statement is true, write true. If the statement is false, change the underlined word or words to make the statement true. 1. _____________ An atom’s valence electrons are those electrons that have the highest energy. 2. _____________ Atoms tend to be stable and nonreactive if they have six va ...
... If the statement is true, write true. If the statement is false, change the underlined word or words to make the statement true. 1. _____________ An atom’s valence electrons are those electrons that have the highest energy. 2. _____________ Atoms tend to be stable and nonreactive if they have six va ...