* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download elements_and_the_periodic_table_2011
Survey
Document related concepts
Transcript
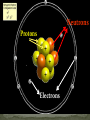
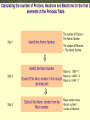
Aristotle and Plato believed there were four elements: Earth, Fire, Air and Water. Their ideas stayed for 2000 year because of their position as philosophers. The elements, working alone or in combinations, make up the entire universe in fact everything around us. Elements are the simplest type of pure substance. Atoms are the smallest part of an element that has all the properties of an element. Gold Element Gold Atom Thompson model Rutherfor Model Bohr Model Wave Model Indivisible Divisible Democritus Aristotle & Plato Dalton Thomson Rutherford Bohr Wave Electron Nucleus Orbit Electron Cloud X X X X X X X X X X X X X X X X Atoms are composed of three types of particles: • Protons: Positively charged particles that are found in the nucleus • Neutron: Uncharged (Neutral) particles that are found in the nucleus • Electrons: negatively charge particles found moving around the nucleus. Neutrons Protons Electrons A space in which electrons are likely to be found. Electrons whirl about the nucleus billions of times in one second They are not moving around in random patterns. Location of electrons depends upon how much energy the electron has. Depending on their energy they are locked into a certain area in the cloud. Electrons with the lowest energy are found in the energy level closest to the nucleus Electrons with the highest energy are found in the outermost energy levels, farther from the nucleus. The nucleus is the core or center of the atom. 99% of the mass. Used to measure the subatomic particles. Protons are 1 amu Neutrons are about 1 amu -27 1 amu = 3.6609 x 10 pounds Electron mass = 0.0005446623 amu Proton mass = 1.00727638 amu Neutron mass = 1.0086649156 amu Region they were found: Magnesium was found in the Greek region Magnesia Greek or Latin word: Lithium came from the Greek word lithos Named for a Scientist, Founder or Country: Einsteinium was named after Albert Einstein. Fermium was named after Enrico Fermi Polonium was named for Poland Each element has a unique symbol First Letter of name (first letter always capitalized): Hydrogen (H) Sulfur (S) Iodine (I) If the first letter was already used or is very common, add another letter from the name (second letter is always lower case): Aluminum (Al), Platinum (Pt), Cadmium (Cd) Some symbols come from their Greek or Latin name: Mercury (Hg) comes from Greek • Elements are defined by the # of protons located inside the atom • Atomic number is the number of protons located in the nucleus. • Atomic Number identifies the element and never changes. All Nitrogen (N) atoms have 7 protons All Carbon (C) atoms have 6 protons All Oxygen (O) atoms have 8 protons Atomic mass is the average mass of all existing isotopes of that element. This is why it is usually not a whole number. An isotope is an atom that has the same number of protons (atomic number) as another atom but a different number of neutrons. Example: C has Isotopes of 12, 13 and 14 All Carbons have 6 protons, but number of neutrons varies. All atoms have a mass number. Mass number is the sum of the protons and neutrons in its nucleus. A molecule is the combination of atoms formed by a covalent bond. (using electrons) A Compound is a substance made up of molecules that contain more than one kind of atom; two or more elements chemically combined.