Chapter 3 Atoms and Elements Classification of Matter Matter Matter
... Isotopes of Sulfur A sample of naturally occurring sulfur contains several isotopes with the following abundances Isotope % abundance 32S ...
... Isotopes of Sulfur A sample of naturally occurring sulfur contains several isotopes with the following abundances Isotope % abundance 32S ...
A Helium atom
... protons and neutrons in the nucleus and electrons in energy levels around the nucleus. Before you can learn how to draw a Bohr model of an atom you must learn a little about the Periodic Table of Elements. ...
... protons and neutrons in the nucleus and electrons in energy levels around the nucleus. Before you can learn how to draw a Bohr model of an atom you must learn a little about the Periodic Table of Elements. ...
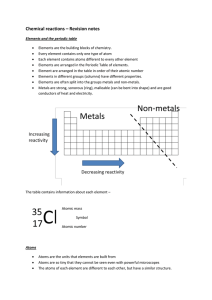
Chemical reactions revision
... Atoms are the units that elements are built from Atoms are so tiny that they cannot be seen even with powerful microscopes The atoms of each element are different to each other, but have a similar structure. ...
... Atoms are the units that elements are built from Atoms are so tiny that they cannot be seen even with powerful microscopes The atoms of each element are different to each other, but have a similar structure. ...
Name________________________________________
... • Located outside of the nucleus in __________or levels called atomic clouds. • Their mass is so small that it is usually considered ___________. • It takes more than 1,800 electrons to equal the mass of one proton. • However electrons occupy most of an atoms ______________. Some things to remember: ...
... • Located outside of the nucleus in __________or levels called atomic clouds. • Their mass is so small that it is usually considered ___________. • It takes more than 1,800 electrons to equal the mass of one proton. • However electrons occupy most of an atoms ______________. Some things to remember: ...
Chapter 4- The Periodic Table Section 1
... John Newland noticed a periodic pattern, Dmitri Mendeleev invented the first periodic table and Henry Moseley found a different physical basis for the arrangement of elements ...
... John Newland noticed a periodic pattern, Dmitri Mendeleev invented the first periodic table and Henry Moseley found a different physical basis for the arrangement of elements ...
U3 Quiz 1: Discovery of the Atom
... a. Atoms cannot be divided, created, or destroyed. b. The number of protons in an atom is its atomic number. c. In chemical reactions, atoms are combined, separated, or rearranged. d. All matter is composed of extremely small particles called atoms. ...
... a. Atoms cannot be divided, created, or destroyed. b. The number of protons in an atom is its atomic number. c. In chemical reactions, atoms are combined, separated, or rearranged. d. All matter is composed of extremely small particles called atoms. ...
Getting to Know: Periodic Table
... Misconception: Why is the atomic mass defined as the “average” mass of atoms of an element? Aren’t all atoms of the same element identical? All atoms of the same element have the same number of protons. All atoms of the same element do not have the same number of neutrons. For example, most atoms of ...
... Misconception: Why is the atomic mass defined as the “average” mass of atoms of an element? Aren’t all atoms of the same element identical? All atoms of the same element have the same number of protons. All atoms of the same element do not have the same number of neutrons. For example, most atoms of ...
Atoms and elements Metals and non-metals
... 2. Work out which group the element is in and draw that number of electrons in the outer circle – with eight for Group 0 elements – except helium. 3. Fill the other circles with as many electrons as needed. Remember – two in the first circle, and eight in the second and third circles. 4. Finally, ch ...
... 2. Work out which group the element is in and draw that number of electrons in the outer circle – with eight for Group 0 elements – except helium. 3. Fill the other circles with as many electrons as needed. Remember – two in the first circle, and eight in the second and third circles. 4. Finally, ch ...
atomic-models
... when passed through an evacuated tube (demos). These rays have a small mass and are negative. Thompson noted that these negative subatomic particles were a fundamental part of all atoms. 1) Dalton’s “Billiard ball” model (1800-1900) Atoms are solid and indivisible. 2) Thompson “Plum pudding” model ( ...
... when passed through an evacuated tube (demos). These rays have a small mass and are negative. Thompson noted that these negative subatomic particles were a fundamental part of all atoms. 1) Dalton’s “Billiard ball” model (1800-1900) Atoms are solid and indivisible. 2) Thompson “Plum pudding” model ( ...
Atoms- Basic Units of Matter
... – Electron cloud: region surrounding an atomic nucleus where an electron is most likely to be found; electrons can be anywhere ...
... – Electron cloud: region surrounding an atomic nucleus where an electron is most likely to be found; electrons can be anywhere ...
Atoms and the Periodic Table
... 3. Elements in columns are called groups or families a. Total of 18 groups b. Groups have same characteristic and # of valence electrons 1. Ve-: electrons in outer shell c. as elements descend atoms grow larger 4. Elements in rows are called series or periods a. elements are arranged by inc. # of pr ...
... 3. Elements in columns are called groups or families a. Total of 18 groups b. Groups have same characteristic and # of valence electrons 1. Ve-: electrons in outer shell c. as elements descend atoms grow larger 4. Elements in rows are called series or periods a. elements are arranged by inc. # of pr ...
Anticipation Guide Before After The periodic table is a collection of
... The atomic number is the number of protons in the nucleus. The atomic number is written below the element on the Periodic Table. The atomic mass is the sum of protons and electrons in the nucleus. The atomic mass is written above the element on the Periodic Table. Atomic mass can be located mostly i ...
... The atomic number is the number of protons in the nucleus. The atomic number is written below the element on the Periodic Table. The atomic mass is the sum of protons and electrons in the nucleus. The atomic mass is written above the element on the Periodic Table. Atomic mass can be located mostly i ...
atom - Images
... How many protons, neutrons, and electrons are present in (a) 27Al3+ (b) 79Se2 Write the isotope notation for an ion that contains 20 protons, 21 neutrons, and 18 electrons. Write the isotope notation for an atom of lead that has 128 neutrons. Write the isotope notation for the following: ...
... How many protons, neutrons, and electrons are present in (a) 27Al3+ (b) 79Se2 Write the isotope notation for an ion that contains 20 protons, 21 neutrons, and 18 electrons. Write the isotope notation for an atom of lead that has 128 neutrons. Write the isotope notation for the following: ...
History of Chemistry
... 1. The nucleus is so small that the alpha particles will go right through. 2. Some will go near gold nuclei and be deflected. ...
... 1. The nucleus is so small that the alpha particles will go right through. 2. Some will go near gold nuclei and be deflected. ...
Periodicity PowerPoint
... remove 1 valence electron. This value is always positive. – First ionization energy is the energy required to remove the first electron – Second ionization energy is the energy required to remove the second electron. It is “always” more energy than the first ionization. – Third ionization energy is ...
... remove 1 valence electron. This value is always positive. – First ionization energy is the energy required to remove the first electron – Second ionization energy is the energy required to remove the second electron. It is “always” more energy than the first ionization. – Third ionization energy is ...
Modern Physics - WordPress.com
... Bohr put the electrons into energy shells around the nucleus Chadwick discovered the neutron in the nucleus The model of the atom took 2000 years to design and we have had it about 70 years! ...
... Bohr put the electrons into energy shells around the nucleus Chadwick discovered the neutron in the nucleus The model of the atom took 2000 years to design and we have had it about 70 years! ...
Periodic Trends
... Atomic size is related the atomic radius of an element, which is defined as half the distance between adjacent nuclei of two atoms of that element. The Trend: Going across the periodic table, atomic radius tends to get smaller. This is because there is an increase of positive charge (more protons), ...
... Atomic size is related the atomic radius of an element, which is defined as half the distance between adjacent nuclei of two atoms of that element. The Trend: Going across the periodic table, atomic radius tends to get smaller. This is because there is an increase of positive charge (more protons), ...
Study Guide 1st Semester
... atoms the way they did? What were their respective models of the atom as a result? 39. What is the difference between an electron in the ground state and excited state? What does this have to do with atomic emission spectra? 40. What are the three forms of radioactive decay? 41. What are the decay e ...
... atoms the way they did? What were their respective models of the atom as a result? 39. What is the difference between an electron in the ground state and excited state? What does this have to do with atomic emission spectra? 40. What are the three forms of radioactive decay? 41. What are the decay e ...
Chemistry Essay - Properties of atoms 21.8Kb
... Since ancient Greek, scientists have been making continuous efforts to understand the true nature of mater. One of the most important achievements that have been made is the periodic table which not only organizes known information, but also enables scientists to predict unknown properties (Hinchlif ...
... Since ancient Greek, scientists have been making continuous efforts to understand the true nature of mater. One of the most important achievements that have been made is the periodic table which not only organizes known information, but also enables scientists to predict unknown properties (Hinchlif ...