ATOMIC STRUCTURE
... called __________ (atoms) In 1803, _______________ studied experiments and concluded that the properties of matter could be explained in terms of __________. Dalton’s _________________ was based on the following ideas: o Each __________ is composed of extremely small particles called atoms. o Al ...
... called __________ (atoms) In 1803, _______________ studied experiments and concluded that the properties of matter could be explained in terms of __________. Dalton’s _________________ was based on the following ideas: o Each __________ is composed of extremely small particles called atoms. o Al ...
notes: 12 - wvhs.wlwv.k12.or.us
... Rutherford’s “Scattering” Experiment: -positively charged alpha particles (helium nuclei) were shot through a thin gold foil. -most alpha particles passed through the foil, or were deflected through moderate angles. -a few were reflected at extreme angles, or even came shooting right back to the sou ...
... Rutherford’s “Scattering” Experiment: -positively charged alpha particles (helium nuclei) were shot through a thin gold foil. -most alpha particles passed through the foil, or were deflected through moderate angles. -a few were reflected at extreme angles, or even came shooting right back to the sou ...
Regents Review Sheet1-3
... Excited State - If the atom absorbs energy, the electrons become “excited” and may jump up to a higher energy level Orbitals - The space within an atom where an electron or pair of electrons is likely to be found Valence Electrons - Electrons in the outermost principal energy level (also called shel ...
... Excited State - If the atom absorbs energy, the electrons become “excited” and may jump up to a higher energy level Orbitals - The space within an atom where an electron or pair of electrons is likely to be found Valence Electrons - Electrons in the outermost principal energy level (also called shel ...
ElementsPeriodicTable Notes
... An atom is composed of positively charged protons, neutral neutrons, and negatively charged electrons. Protons and neutrons are about equal in mass. An electron has about 1/2,000 the mass of a proton or neutron. ...
... An atom is composed of positively charged protons, neutral neutrons, and negatively charged electrons. Protons and neutrons are about equal in mass. An electron has about 1/2,000 the mass of a proton or neutron. ...
Properties of Metals vs. Nonmetals vs. Metalloids
... Energy levels (n=1, 2, 3, 4,…) – represented by periods on the periodic table Sublevels: (s, p, d, f) – represented by blocks on the periodic table Orbitals – region of space where up to 2 electrons may be found ...
... Energy levels (n=1, 2, 3, 4,…) – represented by periods on the periodic table Sublevels: (s, p, d, f) – represented by blocks on the periodic table Orbitals – region of space where up to 2 electrons may be found ...
Chemistry--Chapter 5: Atomic Structure and the Periodic Table
... A. Early Models of the Atom 1. Democritus a. 400 BC, first suggested the existence of indivisible atoms b. No research, no experimental support 2. John Dalton a. late 1700’s conducted research and experiments b. result was Dalton’s atomic theory: 1) All elements are composed of tiny indivisible part ...
... A. Early Models of the Atom 1. Democritus a. 400 BC, first suggested the existence of indivisible atoms b. No research, no experimental support 2. John Dalton a. late 1700’s conducted research and experiments b. result was Dalton’s atomic theory: 1) All elements are composed of tiny indivisible part ...
Name
... 7. Who discovered that elements of the same element have the same number of protons in the nucleus? ______ 8. Who did his research with the gold foil experiment?_________________________________ 9. Who released a paper in which he presented the Law of Constant Composition? ______________________ 10. ...
... 7. Who discovered that elements of the same element have the same number of protons in the nucleus? ______ 8. Who did his research with the gold foil experiment?_________________________________ 9. Who released a paper in which he presented the Law of Constant Composition? ______________________ 10. ...
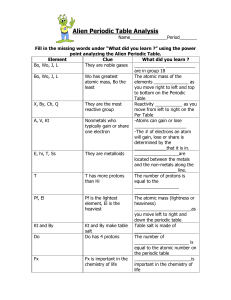
Alien Periodic Table Analysis
... They are the most Reactivity ___________ as you reactive group move from left to right on the Per Table A, V, Kt Nonmetals who -Atoms can gain or lose typically gain or share _____________________ one electron -The # of electrons an atom will gain, lose or share is determined by the _____________tha ...
... They are the most Reactivity ___________ as you reactive group move from left to right on the Per Table A, V, Kt Nonmetals who -Atoms can gain or lose typically gain or share _____________________ one electron -The # of electrons an atom will gain, lose or share is determined by the _____________tha ...
Topic 3&4 Atoms and the per.table
... Standard Grade Revision Units 3 and 4 Q1. The box below shows the names of some elements. ...
... Standard Grade Revision Units 3 and 4 Q1. The box below shows the names of some elements. ...
Atomic Information
... • The size of the charge is easily calculated. Net charge equals protons minus electrons. ...
... • The size of the charge is easily calculated. Net charge equals protons minus electrons. ...
Name Test Review Chapters 4 and 25 Honors Chemistry 1. Fill in
... Created the first atomic theory based on experimental evidence. ___________________________ Discovered the electron. __________________________ Discovered the nucleus. ___________________________ Discovered the Law of Conservation of Mass. __________________________ Proposed a model of the atom as a ...
... Created the first atomic theory based on experimental evidence. ___________________________ Discovered the electron. __________________________ Discovered the nucleus. ___________________________ Discovered the Law of Conservation of Mass. __________________________ Proposed a model of the atom as a ...
NS 4.1 Atoms and Ions
... During chemical reactions, atoms can lose or gain electrons. In fact they do so on a very regular basis. (Atoms only lose or gain protons and neutrons only during nuclear reactions.) Since electrons are negatively charged, when electron(s) are lost, an atom turns into an ion and ends up with a posit ...
... During chemical reactions, atoms can lose or gain electrons. In fact they do so on a very regular basis. (Atoms only lose or gain protons and neutrons only during nuclear reactions.) Since electrons are negatively charged, when electron(s) are lost, an atom turns into an ion and ends up with a posit ...
Name - cloudfront.net
... Identify each element’s place in the periodic table based on these properties and relationships. ...
... Identify each element’s place in the periodic table based on these properties and relationships. ...
Atoms: The Building Blocks of Matter
... Structure of the Atom • Atoms consist of two regions: 1.Nucleus: Which is a very small region located in the center of an atom which contain positively (+) charged particles called protons and one or more (=) neutral particles called neutrons. 2.Electron cloud: A region very large compared to the n ...
... Structure of the Atom • Atoms consist of two regions: 1.Nucleus: Which is a very small region located in the center of an atom which contain positively (+) charged particles called protons and one or more (=) neutral particles called neutrons. 2.Electron cloud: A region very large compared to the n ...
Inside the Atom
... We defined an element as a substance that can not be broken down any more than it already is. This is because elements contain only one type of atom. Is it really impossible to then break down an element? It was until the discovery of nuclear reactions, but even before this scientists were discoveri ...
... We defined an element as a substance that can not be broken down any more than it already is. This is because elements contain only one type of atom. Is it really impossible to then break down an element? It was until the discovery of nuclear reactions, but even before this scientists were discoveri ...
Chapter 2 Test Review - Mercer Island School District
... created or destroyed during a chemical reaction. Matter can only be rearranged. 2AgCl + Na2SO4 Ag2SO4 + 2NaCl ...
... created or destroyed during a chemical reaction. Matter can only be rearranged. 2AgCl + Na2SO4 Ag2SO4 + 2NaCl ...
Atoms, Isotopes, and Ions
... You have learned that atoms contain three smaller particles called protons, neutrons, and electrons, and that the number of protons determines the type of atom. How can you figure out how many neutrons an atom contains, and whether it is neutral or has a charge? Once you know how many protons and ne ...
... You have learned that atoms contain three smaller particles called protons, neutrons, and electrons, and that the number of protons determines the type of atom. How can you figure out how many neutrons an atom contains, and whether it is neutral or has a charge? Once you know how many protons and ne ...
Ch. 2 The Chemical Basis of Life
... The Distribution of Electrons Determines an Atoms Chemical Properties Electrons occupy up to seven electron shells (energy levels) around nucleus Octet rule: Except for the first shell which is full with two electrons, atoms interact in order to have eight electrons in their outermost energy le ...
... The Distribution of Electrons Determines an Atoms Chemical Properties Electrons occupy up to seven electron shells (energy levels) around nucleus Octet rule: Except for the first shell which is full with two electrons, atoms interact in order to have eight electrons in their outermost energy le ...
Atomic number
... How many protons does Calcium have? What element has 17 protons and 18 neutrons? What is its atomic number? What is its atomic mass? ...
... How many protons does Calcium have? What element has 17 protons and 18 neutrons? What is its atomic number? What is its atomic mass? ...
atom
... --Matter is made of small, indivisible particles – “atomos” In the 1700’s, scientists making accurate measurements discovered several new laws -- ...
... --Matter is made of small, indivisible particles – “atomos” In the 1700’s, scientists making accurate measurements discovered several new laws -- ...
Atomic Structure powerpoint
... Says that atoms tend to gain, lose or share electrons so as to have eight electrons in their outer electron shell… More specifically, the number of electrons needed to fill the s and p sublevels of that energy level ...
... Says that atoms tend to gain, lose or share electrons so as to have eight electrons in their outer electron shell… More specifically, the number of electrons needed to fill the s and p sublevels of that energy level ...
Periodic Table
... a. Cations form when an atom gains electrons. b. Cations form when an atom loses electrons. c. Anions form when an atom gains protons. d. Anions form when an atom loses protons. ____ 29. The metals in Groups 1A, 2A, and 3A ____. a. gain electrons when they form ions c. all have ions with a 1 charge ...
... a. Cations form when an atom gains electrons. b. Cations form when an atom loses electrons. c. Anions form when an atom gains protons. d. Anions form when an atom loses protons. ____ 29. The metals in Groups 1A, 2A, and 3A ____. a. gain electrons when they form ions c. all have ions with a 1 charge ...