Packet 5
... Protons – Positive charge, found in the nucleus and have a mass of 1 amu. ( Identify) Neutrons- No charge, found in the nucleus, and have a mass of 1 amu ( Isotopes) Electrons- Negative charge, found in the energy levels outside of the nucleus, have relatively no mass ( Ions) ...
... Protons – Positive charge, found in the nucleus and have a mass of 1 amu. ( Identify) Neutrons- No charge, found in the nucleus, and have a mass of 1 amu ( Isotopes) Electrons- Negative charge, found in the energy levels outside of the nucleus, have relatively no mass ( Ions) ...
Nuclear Chemistry Worksheet
... AMU: 1 amu is defined as 1/12 the mass of a Carbon-12 atom. ***If you are given the atomic mass, you can subtract the atomic number to find the number of neutrons!*** In a neutral atom, i.e. an atom that does not have a charge overall, the number of protons equals the number of electrons!! Isotopes ...
... AMU: 1 amu is defined as 1/12 the mass of a Carbon-12 atom. ***If you are given the atomic mass, you can subtract the atomic number to find the number of neutrons!*** In a neutral atom, i.e. an atom that does not have a charge overall, the number of protons equals the number of electrons!! Isotopes ...
periodic table
... First is Tc (technetium: greek for artificial) and all elements > 92 (plus 61) 9. Halogens: Fluorine group: 17, very reactive due to -1 oxidation # (7 e- in valence shell): combine w/ alkali metals to form salts 10. Alkali metals: Hydrogen group: 1, very reactive due to +1 oxidation # (1e- in the va ...
... First is Tc (technetium: greek for artificial) and all elements > 92 (plus 61) 9. Halogens: Fluorine group: 17, very reactive due to -1 oxidation # (7 e- in valence shell): combine w/ alkali metals to form salts 10. Alkali metals: Hydrogen group: 1, very reactive due to +1 oxidation # (1e- in the va ...
electron
... mass 10.012 amu and a relative abundance of 19.91%. The isotope with mass 11.009 amu has a relative abundance of 80.09%. 1. Calculate the atomic mass of this element (show all work) and then name this element. ...
... mass 10.012 amu and a relative abundance of 19.91%. The isotope with mass 11.009 amu has a relative abundance of 80.09%. 1. Calculate the atomic mass of this element (show all work) and then name this element. ...
Atomic history - Kenton County Schools
... Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3rd Edition, 1990, page 204 ...
... Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3rd Edition, 1990, page 204 ...
AP Chapter 2 Objectives
... Describe how Rutherford’s gold foil experiment established the basic structure of the nuclear atom. 2.3 The Modern View of Atomic Structure I can… ...
... Describe how Rutherford’s gold foil experiment established the basic structure of the nuclear atom. 2.3 The Modern View of Atomic Structure I can… ...
Elements and the Periodic Table
... 9. What term is used to describe all the elements that are in the same vertical column in the periodic table? ...
... 9. What term is used to describe all the elements that are in the same vertical column in the periodic table? ...
Atoms & Elements
... Let’s look at pure substances? • matter with a specific composition. • an element when composed of one type of atom. Elements are • pure substances that contains atoms of only one type. ...
... Let’s look at pure substances? • matter with a specific composition. • an element when composed of one type of atom. Elements are • pure substances that contains atoms of only one type. ...
Test review
... have an outer electron configuration of s1 and form 1+ ions when stable 23. group of elements on the periodic table that have an outer electron configuration of s2p4 and form 2- ions when stable 24. group of elements on the periodic table that have an outer electron configuration of s2p3 and form 3- ...
... have an outer electron configuration of s1 and form 1+ ions when stable 23. group of elements on the periodic table that have an outer electron configuration of s2p4 and form 2- ions when stable 24. group of elements on the periodic table that have an outer electron configuration of s2p3 and form 3- ...
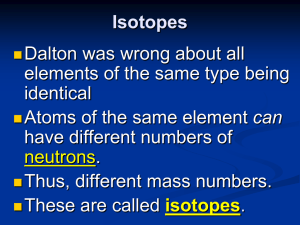
Isotopes
... It depends, because there are different kinds of oxygen atoms. We are more concerned with the average atomic mass. This is based on the abundance (percentage) of each variety of that element in nature. ...
... It depends, because there are different kinds of oxygen atoms. We are more concerned with the average atomic mass. This is based on the abundance (percentage) of each variety of that element in nature. ...
CH 2 Linear
... Early Ideas: Greek Philosophers (2500 years ago) only observed matter, but did not test their hypotheses with experiments... No scientific model/method was followed. Example: Piece of aluminum foil. How small can one cut the pieces of foil and still have aluminum? If I continue to cut, will I no ...
... Early Ideas: Greek Philosophers (2500 years ago) only observed matter, but did not test their hypotheses with experiments... No scientific model/method was followed. Example: Piece of aluminum foil. How small can one cut the pieces of foil and still have aluminum? If I continue to cut, will I no ...
Basic Chemistry Lesson - Agriculture Solutions
... Atoms are the building blocks of everything. An atom consists of a central nucleus, containing protons [positively charged] and neutrons [no charge] that is usually surrounded by one or more electrons [negatively charged] orbiting the nucleus in layers or“shells”. See the simplified diagram on the l ...
... Atoms are the building blocks of everything. An atom consists of a central nucleus, containing protons [positively charged] and neutrons [no charge] that is usually surrounded by one or more electrons [negatively charged] orbiting the nucleus in layers or“shells”. See the simplified diagram on the l ...
Matter on Earth and in the universe is made of atoms that have
... 3. What is the most abundant element in the universe? Where do all the other elements come from? What is the element of life? Hydrogen is the most abundant element in the universe. See question 1 for an explanation of where other elements come from. Carbon is the element of life. 4. Light elements a ...
... 3. What is the most abundant element in the universe? Where do all the other elements come from? What is the element of life? Hydrogen is the most abundant element in the universe. See question 1 for an explanation of where other elements come from. Carbon is the element of life. 4. Light elements a ...
Atoms, Molecules and Ions
... For example, naturally occurring carbon, for example, is a mixture of two isotopes, 12C (98.89%) and 13C (1.11 %). Individual carbon atoms therefore have a mass of either 12.000 or 13.03354 amu. But the average mass of the different isotopes of carbon is 12.011 amu. ...
... For example, naturally occurring carbon, for example, is a mixture of two isotopes, 12C (98.89%) and 13C (1.11 %). Individual carbon atoms therefore have a mass of either 12.000 or 13.03354 amu. But the average mass of the different isotopes of carbon is 12.011 amu. ...
History of Atomic Theory
... where an electron is at the same time. In other words, you can find out where the electron started and you can see where the electron ended up but how it got there WE DON'T KNOW! ...
... where an electron is at the same time. In other words, you can find out where the electron started and you can see where the electron ended up but how it got there WE DON'T KNOW! ...
Trends in the Periodic Table
... 1. __________________ An atom of this element has 1 proton and 1 electron 2. __________________ An atom of this element has 8 protons 3. __________________ An atom of this element has 10 more protons than an atom of oxygen 4. __________________ This element has an atomic number of 12 5. ____________ ...
... 1. __________________ An atom of this element has 1 proton and 1 electron 2. __________________ An atom of this element has 8 protons 3. __________________ An atom of this element has 10 more protons than an atom of oxygen 4. __________________ This element has an atomic number of 12 5. ____________ ...
Chapter 2: Matter is Made up of Atoms
... • In 1910 Thomson discovered that neon atoms have different masses. • In 1932, James Chadwick confirms existence of the neutron • Conclusion: there must be another particle that has no charge, called a neutron. ...
... • In 1910 Thomson discovered that neon atoms have different masses. • In 1932, James Chadwick confirms existence of the neutron • Conclusion: there must be another particle that has no charge, called a neutron. ...
Inside the Atom
... A New Atomic Model • Since electrons have no mass and there are no other particles, the mass of an atom must equal the number of protons • The mass of atoms, however, is at least twice that of the number of protons • It was proposed that another particle must be in the nucleus • Later called the ne ...
... A New Atomic Model • Since electrons have no mass and there are no other particles, the mass of an atom must equal the number of protons • The mass of atoms, however, is at least twice that of the number of protons • It was proposed that another particle must be in the nucleus • Later called the ne ...
Periodic Table WebQuest
... 9. Is the quantity of protons always equal to the quantity of neutrons in an atom? Explain. 10. List 3 uses of isotopes. 11. Describe how scientists calculate the average atomic mass of an element. Use http://www.chem4kids.com/files/elem_intro.html, http://www.chem4kids.com/files/elem_transmetal.ht ...
... 9. Is the quantity of protons always equal to the quantity of neutrons in an atom? Explain. 10. List 3 uses of isotopes. 11. Describe how scientists calculate the average atomic mass of an element. Use http://www.chem4kids.com/files/elem_intro.html, http://www.chem4kids.com/files/elem_transmetal.ht ...
DEVELOPMENT OF THE ATOMIC MODEL
... left several “holes” in his table and occasionally reversed the order of elements to fit the properties of others in that column The “holes” were later filled in with newly discovered elements that had the properties predicted by Mendeleev’s table. The reason for the reversal of elements was explain ...
... left several “holes” in his table and occasionally reversed the order of elements to fit the properties of others in that column The “holes” were later filled in with newly discovered elements that had the properties predicted by Mendeleev’s table. The reason for the reversal of elements was explain ...
atoms
... But first….THEORY • What is a theory in science? • A theory is a well-tested explanation of what happens in nature. • In layman’s terms, if something is said to be “just a theory,” it usually means that it is a mere guess, or is unproven. But in scientific terms, a theory implies that something has ...
... But first….THEORY • What is a theory in science? • A theory is a well-tested explanation of what happens in nature. • In layman’s terms, if something is said to be “just a theory,” it usually means that it is a mere guess, or is unproven. But in scientific terms, a theory implies that something has ...
UNIT 1 EXAM REVIEW Scientific Method What are the steps in the
... Molecule is more than one atom bonded together. H2, H2O, NaCl 47. What is a mixture? Give 2 examples of mixtures. Two elements or compounds that are not bonded together (NaCl and H 2O) (N2 and O2) 48. Give some examples of physical changes. Water changing states, a piece of paper being crumpled, but ...
... Molecule is more than one atom bonded together. H2, H2O, NaCl 47. What is a mixture? Give 2 examples of mixtures. Two elements or compounds that are not bonded together (NaCl and H 2O) (N2 and O2) 48. Give some examples of physical changes. Water changing states, a piece of paper being crumpled, but ...