Isotopes and Average Atomic Mass
... higher energy orbit of larger radius. (excited electrons) An excited electron can fall back to its original orbit by emitting energy as radiation. Electrons can only exist in certain discrete energy levels. ...
... higher energy orbit of larger radius. (excited electrons) An excited electron can fall back to its original orbit by emitting energy as radiation. Electrons can only exist in certain discrete energy levels. ...
Notes - Learner
... As we go away from the nucleus, the energy levels come closer, i.e., with the increase in the value of n, the difference of energy between successive orbits decreases. ...
... As we go away from the nucleus, the energy levels come closer, i.e., with the increase in the value of n, the difference of energy between successive orbits decreases. ...
Build an Atom
... Protons have a relative mass of ___________ amu and a charge of ___________. Neutrons have a relative mass of ___________ amu and a charge of ___________. Electrons have a relative mass of nearly___________ amu and a charge of ___________. ...
... Protons have a relative mass of ___________ amu and a charge of ___________. Neutrons have a relative mass of ___________ amu and a charge of ___________. Electrons have a relative mass of nearly___________ amu and a charge of ___________. ...
ATOMS: THE BUILDING BLOCKS OF MATTER from the
... 1. ____________ are connected to ____________ because these two types of sub-atomic particles are in the nucleus of atoms. 2. ____________ is NOT connected to ____________ because one refers to the mass of an element and the other refers to the number of protons in its nucleus. 3. An ____________ is ...
... 1. ____________ are connected to ____________ because these two types of sub-atomic particles are in the nucleus of atoms. 2. ____________ is NOT connected to ____________ because one refers to the mass of an element and the other refers to the number of protons in its nucleus. 3. An ____________ is ...
PHS 004lecture1
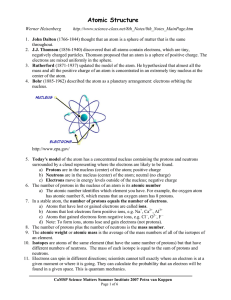
... pudding filled with a positively charged material. • Rutherford concluded that an atom had a small, dense, positively charged center that repelled his ...
... pudding filled with a positively charged material. • Rutherford concluded that an atom had a small, dense, positively charged center that repelled his ...
Atoms and the Periodic Table
... successful than Democritus's theory because it had a scientific basis. ...
... successful than Democritus's theory because it had a scientific basis. ...
Webquest - TeacherWeb
... 2. In what date was it determined that matter can neither be created nor destroyed. Name the date and the scientist. 3. Name the date and inventor of the modern version of the Atomic Theory. 4. I was born in 1831 and showed that electricity and magnetism are scientifically related. 5. He developed t ...
... 2. In what date was it determined that matter can neither be created nor destroyed. Name the date and the scientist. 3. Name the date and inventor of the modern version of the Atomic Theory. 4. I was born in 1831 and showed that electricity and magnetism are scientifically related. 5. He developed t ...
Atomic Mass
... radioactive methods, ought to begin a small research?" Now I had thought that, too, so I said, " Why not let him see if any alpha-particles can be scattered through a large angle?" I may tell you in confidence that I did not believe that they would be, since we knew the alpha-particle was a very fas ...
... radioactive methods, ought to begin a small research?" Now I had thought that, too, so I said, " Why not let him see if any alpha-particles can be scattered through a large angle?" I may tell you in confidence that I did not believe that they would be, since we knew the alpha-particle was a very fas ...
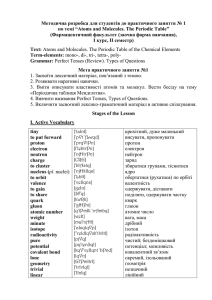
по темі “Atoms and Molecules. The Periodic Table”
... 1. __________________ – a table of the elements, arranged in order of increasing atomic number, based on the periodic law. 2. ______________ – the inventor of the periodic table of the chemical elements. 3. ______________ – a transuranic element artificially produced by bombardment of Einsteinium. S ...
... 1. __________________ – a table of the elements, arranged in order of increasing atomic number, based on the periodic law. 2. ______________ – the inventor of the periodic table of the chemical elements. 3. ______________ – a transuranic element artificially produced by bombardment of Einsteinium. S ...
Chapter 5 Powerpoint
... a physical model. However, not all models are physical. In fact, several theoretical models of the atom have been developed over the last few hundred years. You will learn about the currently accepted model of how electrons behave in atoms. ...
... a physical model. However, not all models are physical. In fact, several theoretical models of the atom have been developed over the last few hundred years. You will learn about the currently accepted model of how electrons behave in atoms. ...
History of the Atom Model
... Not only did Bohr predict that electrons would occupy specific energy levels, he also predicted that those levels had limits to the number of electrons each could hold. Under Bohr's theory, the maximum capacity of the first (or innermost) electron shell is two electrons. For any element with more th ...
... Not only did Bohr predict that electrons would occupy specific energy levels, he also predicted that those levels had limits to the number of electrons each could hold. Under Bohr's theory, the maximum capacity of the first (or innermost) electron shell is two electrons. For any element with more th ...
IT`S ATOMIC
... in their chemical symbols. If the chemical symbol is just one letter, that letter is a capital letter. If the symbol has two or more letters, then the first letter is a capital and the following letters are lower case. This standard for writing symbols for the elements makes it easy for scientists t ...
... in their chemical symbols. If the chemical symbol is just one letter, that letter is a capital letter. If the symbol has two or more letters, then the first letter is a capital and the following letters are lower case. This standard for writing symbols for the elements makes it easy for scientists t ...
Bell work: Date - Wando High School
... in the NUMBER OF ELECTRONS they have. To do this, atoms will gain or lose electrons to achieve the same number of electrons as the closest noble gas. ...
... in the NUMBER OF ELECTRONS they have. To do this, atoms will gain or lose electrons to achieve the same number of electrons as the closest noble gas. ...
Periodic Table 1
... arranged the atoms, but he also made predictions based on his arrangements His predictions were later shown to be quite accurate. ...
... arranged the atoms, but he also made predictions based on his arrangements His predictions were later shown to be quite accurate. ...
Know (main topic)
... - -memorize the 3 subatomic particles of an atom -determine atomic number, mass number, protons, electrons, & neutrons from the periodic table -explain why the nucleus of the atom is much smaller than the atom yet contains most of its mass -relate the position of an element in the Periodic Table to ...
... - -memorize the 3 subatomic particles of an atom -determine atomic number, mass number, protons, electrons, & neutrons from the periodic table -explain why the nucleus of the atom is much smaller than the atom yet contains most of its mass -relate the position of an element in the Periodic Table to ...
Atomic Theory Notes- Chapters 5 and 13
... atomic weight if 75.53% of the naturally occurring element is chlorine 35, and 24.47% is chlorine 37. ...
... atomic weight if 75.53% of the naturally occurring element is chlorine 35, and 24.47% is chlorine 37. ...
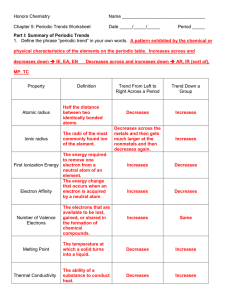
AP CHEMISTRY Periodic Trends Worksheet
... a. How would this account for the trend you discovered in atomic radius? Electrons are held more tightly and are pulled in closer by the nucleus as the number of protons increases within a level. b. How would this account for the trend you discovered in the first ionization energy? Because of the ad ...
... a. How would this account for the trend you discovered in atomic radius? Electrons are held more tightly and are pulled in closer by the nucleus as the number of protons increases within a level. b. How would this account for the trend you discovered in the first ionization energy? Because of the ad ...
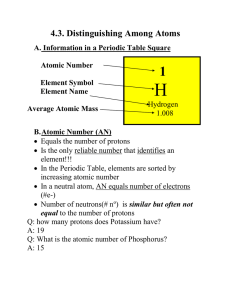
Introduction to Atomic Structure - California K
... surrounded by a cloud representing where the electrons are likely to be found. a) Protons are in the nucleus (center) of the atom; positive charge b) Neutrons are in the nucleus (center) of the atom; neutral (no charge) c) Electrons move in energy levels outside of the nucleus; negative charge 6. Th ...
... surrounded by a cloud representing where the electrons are likely to be found. a) Protons are in the nucleus (center) of the atom; positive charge b) Neutrons are in the nucleus (center) of the atom; neutral (no charge) c) Electrons move in energy levels outside of the nucleus; negative charge 6. Th ...
The Development of the Atomic Theory
... Same size, mass and chemical properties Atoms are indivisible in chemical processes. They can neither be created nor destroyed in a chemical reaction A chemical reaction simply changes the way the atom is grouped together A compound has a constant composition of its elements Different atoms ...
... Same size, mass and chemical properties Atoms are indivisible in chemical processes. They can neither be created nor destroyed in a chemical reaction A chemical reaction simply changes the way the atom is grouped together A compound has a constant composition of its elements Different atoms ...
Notes 4.3 filled in
... a) All the element’s existing isotopes and their masses b) their natural abundance (frequency in nature) ...
... a) All the element’s existing isotopes and their masses b) their natural abundance (frequency in nature) ...
Chem midterm review powerpoint
... An element is a pure substance that cannot be separated into simpler substances by physical or chemical means. • 92 elements occur naturally on Earth. • Each element has a unique name and a one, two, or three-letter symbol. • The periodic table organizes the elements into a grid of horizontal rows c ...
... An element is a pure substance that cannot be separated into simpler substances by physical or chemical means. • 92 elements occur naturally on Earth. • Each element has a unique name and a one, two, or three-letter symbol. • The periodic table organizes the elements into a grid of horizontal rows c ...