PPT Atoms and Periodicity
... more energy to remove each electron IE1 < IE2 < IE3, … look for a huge jump in IE once all valence e–’s are removed, the next e– is on an inner level with attraction (shielding & Zeff). huge jump in IE4 b/c 4th e– on inner level (must have 3 valence e–’s) ...
... more energy to remove each electron IE1 < IE2 < IE3, … look for a huge jump in IE once all valence e–’s are removed, the next e– is on an inner level with attraction (shielding & Zeff). huge jump in IE4 b/c 4th e– on inner level (must have 3 valence e–’s) ...
J.E. Strong, Jr. 1/21/2012 How big is a Hydrogen Atom? It is very
... reference. Let’s look at how large a simple hydrogen atom is by comparing it to something more familiar. What is hydrogen (and why do we care)? Hydrogen is the simplest chemical element, with an atomic number of 1, meaning its nucleus consists of a single proton. In its normal state (a gas), a hydro ...
... reference. Let’s look at how large a simple hydrogen atom is by comparing it to something more familiar. What is hydrogen (and why do we care)? Hydrogen is the simplest chemical element, with an atomic number of 1, meaning its nucleus consists of a single proton. In its normal state (a gas), a hydro ...
atom
... Atoms of same element have the same size, mass, and properties Atoms can’t be subdivided, created or destroyed Atoms of different elements combine in whole number ratios to make compounds In chemical reactions, atoms can be combined, separated, and rearranged. ...
... Atoms of same element have the same size, mass, and properties Atoms can’t be subdivided, created or destroyed Atoms of different elements combine in whole number ratios to make compounds In chemical reactions, atoms can be combined, separated, and rearranged. ...
Electron Arrangements - Madison Public Schools
... 1. I.E. increases as you go from left to right on the periodic table Why? • Increasing nuclear charge as you move to the right; the nucleus has a stronger attraction for the outer electrons 2. I.E increases as you move up a group on the periodic table. Why? • Electrons are closer to the nucleus as y ...
... 1. I.E. increases as you go from left to right on the periodic table Why? • Increasing nuclear charge as you move to the right; the nucleus has a stronger attraction for the outer electrons 2. I.E increases as you move up a group on the periodic table. Why? • Electrons are closer to the nucleus as y ...
Chapter 11: The Atomic Nature of Matter
... • Uncharged particles in the neutron, with mass ~ that of proton. • The # of neutrons need not match # of protons in atom, eg. H typically has 1 proton and 0 neutrons, but some H atoms may have 1 neutron, but always 1 proton, (called “heavy hydrogen”) • Isotopes = atoms of same element that contain ...
... • Uncharged particles in the neutron, with mass ~ that of proton. • The # of neutrons need not match # of protons in atom, eg. H typically has 1 proton and 0 neutrons, but some H atoms may have 1 neutron, but always 1 proton, (called “heavy hydrogen”) • Isotopes = atoms of same element that contain ...
Unit 3 GROUP QUIZ
... b. Electrons are negatively charged and have a mass of 1 u. c. The nucleus of an atom is positively charged. d. The neutron is found in the nucleus of an atom. ___11. All atoms are ______. a. positively charged, with the number of protons exceeding the number of electrons. b. Negatively charged, wit ...
... b. Electrons are negatively charged and have a mass of 1 u. c. The nucleus of an atom is positively charged. d. The neutron is found in the nucleus of an atom. ___11. All atoms are ______. a. positively charged, with the number of protons exceeding the number of electrons. b. Negatively charged, wit ...
Atomic Mass
... Electrons orbit the nucleus in orbits that have a set size and energy. The energy of the orbit is related to its size. The lowest energy is found in the smallest orbit. Radiation is absorbed or emitted when an electron moves from one orbit to another. Problems: We cannot predict the exact location ...
... Electrons orbit the nucleus in orbits that have a set size and energy. The energy of the orbit is related to its size. The lowest energy is found in the smallest orbit. Radiation is absorbed or emitted when an electron moves from one orbit to another. Problems: We cannot predict the exact location ...
Thomson`s Experiment
... How Rutherford Explained It To explain the results of the experiment, Rutherford’s team proposed a new model of the atom: Because most of the particles passed through the foil, they concluded that the atom is nearly all empty space. ...
... How Rutherford Explained It To explain the results of the experiment, Rutherford’s team proposed a new model of the atom: Because most of the particles passed through the foil, they concluded that the atom is nearly all empty space. ...
Nature of Molecules and Water
... Hydrogen Bonds and Water • Single most outstanding chemical property of water is its ability to form hydrogen bonds – Weak chemical associations that form between the partially negative O atoms and the partially positive H atoms of two water molecules • Each individual bond is weak • Cumulative ef ...
... Hydrogen Bonds and Water • Single most outstanding chemical property of water is its ability to form hydrogen bonds – Weak chemical associations that form between the partially negative O atoms and the partially positive H atoms of two water molecules • Each individual bond is weak • Cumulative ef ...
Chapter 4 Review Packet Section 4.1
... •Reasoned that the atom was indivisible and indestructable •Democritus ideas agreed with scientific theory, but did not include chemical behavior and had a lack of experimental support. John Dalton •By using experimental methods, Dalton transformed Democritus's ideas on atoms into scientific theory ...
... •Reasoned that the atom was indivisible and indestructable •Democritus ideas agreed with scientific theory, but did not include chemical behavior and had a lack of experimental support. John Dalton •By using experimental methods, Dalton transformed Democritus's ideas on atoms into scientific theory ...
Chapter 3—Time and Geology
... elsewhere in Europe that were higher (hence, younger according to superposition) than those described as Silurian in southern Wales by Murchison. FIGURE 3–7 (p. 39) The stratum beneath the ash must be older than 453.7 million years. FIGURE 3–10 (p. 40) If a graph were prepared showing how much sand ...
... elsewhere in Europe that were higher (hence, younger according to superposition) than those described as Silurian in southern Wales by Murchison. FIGURE 3–7 (p. 39) The stratum beneath the ash must be older than 453.7 million years. FIGURE 3–10 (p. 40) If a graph were prepared showing how much sand ...
CHEM_S1CourseReview_2011
... What are the components of a good scientific experiment? What rules must be obeyed to safely conduct an experiment? Why are significant figures important to chemists? What is the best method/graph to represent specific data? How would a scientist organize data collected from an experiment ...
... What are the components of a good scientific experiment? What rules must be obeyed to safely conduct an experiment? Why are significant figures important to chemists? What is the best method/graph to represent specific data? How would a scientist organize data collected from an experiment ...
Notes: Unit 3: Atomic Concepts - Mr. Palermo`s Flipped Chemistry
... 4. Determine the number of protons, neutrons, and electrons in an ion 5. Identify the subatomic particles of an atom (proton, neutron, and electron) 6. Determine the number of protons, neutrons, electrons, nucleons and nuclear charge in a neutral atom 7. Differentiate between atomic number, mass num ...
... 4. Determine the number of protons, neutrons, and electrons in an ion 5. Identify the subatomic particles of an atom (proton, neutron, and electron) 6. Determine the number of protons, neutrons, electrons, nucleons and nuclear charge in a neutral atom 7. Differentiate between atomic number, mass num ...
Campbell Biology, 10e (Reece) Chapter 2 The Chemical Context of
... 5) Knowing the atomic mass of an element allows inferences about which of the following? A) the number of electrons in the element B) the number of protons in the element C) the number of protons plus neutrons in the element D) the number of protons plus electrons in the element 6) In what way are ...
... 5) Knowing the atomic mass of an element allows inferences about which of the following? A) the number of electrons in the element B) the number of protons in the element C) the number of protons plus neutrons in the element D) the number of protons plus electrons in the element 6) In what way are ...
atom
... in certain proportions based on mass to form compounds. John Dalton, a British chemist and schoolteacher, wanted to know why. He experimented with different substances. His results suggested that elements combine in certain proportions because they are made of single atoms. Dalton published his atom ...
... in certain proportions based on mass to form compounds. John Dalton, a British chemist and schoolteacher, wanted to know why. He experimented with different substances. His results suggested that elements combine in certain proportions because they are made of single atoms. Dalton published his atom ...
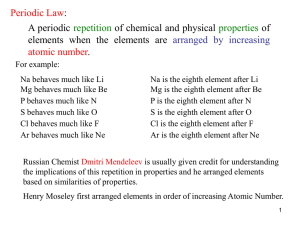
The periodic table
... • For example, he predicted the properties of an undiscovered element that should fit below aluminium in his table. When this element, called gallium, was discovered in 1875 its properties were found to be close to Mendeleev's predictions. Two other predicted elements were later discovered, lending ...
... • For example, he predicted the properties of an undiscovered element that should fit below aluminium in his table. When this element, called gallium, was discovered in 1875 its properties were found to be close to Mendeleev's predictions. Two other predicted elements were later discovered, lending ...
Increasing Radii
... Within each block label each column as 1, 2, 3 ….. (for example which column would be s1, p3, d6, or f2). Choose an element within each block and write out an electron configuration for that element (use the noble gas shortcut method). Choose an element from each s or p column and write a dot diagra ...
... Within each block label each column as 1, 2, 3 ….. (for example which column would be s1, p3, d6, or f2). Choose an element within each block and write out an electron configuration for that element (use the noble gas shortcut method). Choose an element from each s or p column and write a dot diagra ...
U2notes2015
... 3. Dalton’s Law of multiple proportions: when 2 elements form a series of compounds, the ratios of the masses of nd the 2 element that combine with 1 gram of the first element can always be reduced to small whole numbers. ...
... 3. Dalton’s Law of multiple proportions: when 2 elements form a series of compounds, the ratios of the masses of nd the 2 element that combine with 1 gram of the first element can always be reduced to small whole numbers. ...
Coloring the Periodic Table - Families
... Elements in Group 16 only need two more electrons to fill their outer level. Elements in Group 17 only need one more electron to fill their outer level. ...
... Elements in Group 16 only need two more electrons to fill their outer level. Elements in Group 17 only need one more electron to fill their outer level. ...
The Periodic Table of Elements
... b. It reacts vigorously with water to give off hydrogen. c. It forms the ionic chloride with formula CsCl2 d. It forms an acidic oxide. 17. Which statement is most likely to be true about the elements in Group I of the Periodic ...
... b. It reacts vigorously with water to give off hydrogen. c. It forms the ionic chloride with formula CsCl2 d. It forms an acidic oxide. 17. Which statement is most likely to be true about the elements in Group I of the Periodic ...
element symbol on PT
... 1. Round the bottom number to the nearest whole number 2. Subtract the atomic number from the atomic mass number. (You will always subtract the smaller number on top from the larger bottom number) ...
... 1. Round the bottom number to the nearest whole number 2. Subtract the atomic number from the atomic mass number. (You will always subtract the smaller number on top from the larger bottom number) ...
Lecture 12 pdf
... • Uncharged particles in the neutron, with mass ~ that of proton. • The # of neutrons need not match # of protons in atom, eg. H typically has 1 proton and 0 neutrons, but some H atoms may have 1 neutron, but always 1 proton, (called “heavy hydrogen”) • Isotopes = atoms of same element that contain ...
... • Uncharged particles in the neutron, with mass ~ that of proton. • The # of neutrons need not match # of protons in atom, eg. H typically has 1 proton and 0 neutrons, but some H atoms may have 1 neutron, but always 1 proton, (called “heavy hydrogen”) • Isotopes = atoms of same element that contain ...