Chapter # 4 notes
... The standard to which the masses of all other atoms are compared to was chosen to be the most abundant isotope of carbon. A mass of exactly 12 atomic mass units (amu) was assigned to the carbon-12 atom. An amu is defined as exactly equal to the mass of a carbon-12 atom. 1 amu = 1.6606 x 10-24 g Isot ...
... The standard to which the masses of all other atoms are compared to was chosen to be the most abundant isotope of carbon. A mass of exactly 12 atomic mass units (amu) was assigned to the carbon-12 atom. An amu is defined as exactly equal to the mass of a carbon-12 atom. 1 amu = 1.6606 x 10-24 g Isot ...
How can atomic theory explain patterns in the periodic table?
... new element has been made and what to call it? The International Union of Pure and Applied Chemistry, or IUPAC for short, is the international scientific organization that is in charge of naming chemical elements, as well as other chemicals. They are also in charge of confirming that new elements ha ...
... new element has been made and what to call it? The International Union of Pure and Applied Chemistry, or IUPAC for short, is the international scientific organization that is in charge of naming chemical elements, as well as other chemicals. They are also in charge of confirming that new elements ha ...
Atoms and Elements: Are they Related?
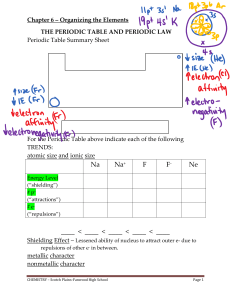
... Periods – Rows are called periods. The elements in these rows change conductivity and number of electrons as you move across the table. Groups – Columns are called groups or families. These elements have the same properties because of the number of electrons. ...
... Periods – Rows are called periods. The elements in these rows change conductivity and number of electrons as you move across the table. Groups – Columns are called groups or families. These elements have the same properties because of the number of electrons. ...
Chapter 4 Presentation - Spearfish School District
... 1. The different colors that were created by using different gases showed that atoms of different elements possessed different energies. 2. The cast shadow was thought to be due to the beam of light created by the cathode-ray. However, the experiment made with the spinning paddle-wheel showed that t ...
... 1. The different colors that were created by using different gases showed that atoms of different elements possessed different energies. 2. The cast shadow was thought to be due to the beam of light created by the cathode-ray. However, the experiment made with the spinning paddle-wheel showed that t ...
DEFINING THE ATOM
... How did Democritus characterize atoms? How did Dalton advance the atomic philosophy proposed by Democritus? What instrument can be used to observe individual atoms? In your own words, state the main ideas of Dalton’s atomic theory. According to Dalton’s atomic theory, is it impossible to convert ato ...
... How did Democritus characterize atoms? How did Dalton advance the atomic philosophy proposed by Democritus? What instrument can be used to observe individual atoms? In your own words, state the main ideas of Dalton’s atomic theory. According to Dalton’s atomic theory, is it impossible to convert ato ...
Atoms, Molecules and Ions
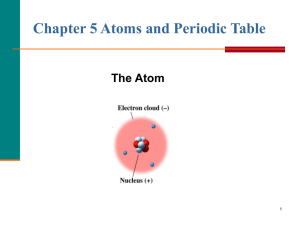
... All matter is made of atoms. These indivisible and indestructible objects are the ultimate chemical particles. All the atoms of a given element are identical, in both weight and chemical properties. However, atoms of different elements have different weights and different chemical properties. Compou ...
... All matter is made of atoms. These indivisible and indestructible objects are the ultimate chemical particles. All the atoms of a given element are identical, in both weight and chemical properties. However, atoms of different elements have different weights and different chemical properties. Compou ...
Name:
... Now, for the “rest” of the story… The Russian scientist Dmitri Medeleev was the first chemist responsible for “creating” the periodic chart of elements. Scientists during Dmitri’s time were trying to figure out an easy way in which they could organize the elements of matter so that it would be easy ...
... Now, for the “rest” of the story… The Russian scientist Dmitri Medeleev was the first chemist responsible for “creating” the periodic chart of elements. Scientists during Dmitri’s time were trying to figure out an easy way in which they could organize the elements of matter so that it would be easy ...
Midterm Review - Closter Public Schools
... An ________________________ is the force of attraction between positive and negatively charged ions. _________________ form positive ions when they _______________ electrons. _________________form negative ions when they ________________ electrons. An ion with a +2 charge has ______________ 2 electr ...
... An ________________________ is the force of attraction between positive and negatively charged ions. _________________ form positive ions when they _______________ electrons. _________________form negative ions when they ________________ electrons. An ion with a +2 charge has ______________ 2 electr ...
Chem Activity: Polyatomic Ions
... 3. One at a time, enter the atomic number for each Group 1 element in the yellow box to observe its atomic radius (see image below). A blue box will appear around the point on the graph corresponding to that element. ...
... 3. One at a time, enter the atomic number for each Group 1 element in the yellow box to observe its atomic radius (see image below). A blue box will appear around the point on the graph corresponding to that element. ...
Chapter 2 slides
... outermost electron • The lower the ionization energy, the easier it is to form a cation ionization energy + Na Na+ + e- ...
... outermost electron • The lower the ionization energy, the easier it is to form a cation ionization energy + Na Na+ + e- ...
vocab - SALAZAR!!
... Alkaline Earth metals are reactive, also, but not as reactive as the Alkali metals. 3. Halogen- The halogens or halogen elements are a group in the periodic table consisting of five chemically related elements, fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and ...
... Alkaline Earth metals are reactive, also, but not as reactive as the Alkali metals. 3. Halogen- The halogens or halogen elements are a group in the periodic table consisting of five chemically related elements, fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and ...
Chapter 6 - HCC Learning Web
... • The periodic table was expanded by one group at the far right of the periodic table with the discovery of argon in 1894. • Helium, neon, krypton, xenon, and radon were subsequently discovered in the next 5 years. • They were originally called the inert gases. • Recently, several compounds of xenon ...
... • The periodic table was expanded by one group at the far right of the periodic table with the discovery of argon in 1894. • Helium, neon, krypton, xenon, and radon were subsequently discovered in the next 5 years. • They were originally called the inert gases. • Recently, several compounds of xenon ...
Atomic Basics
... Although Neils Bohr put the electrons into orbits, which was wrong, he did put them into energy levels. These levels are exact, and the electrons tend to stay in the lowest energy levels possible. That means that the orbitals (his orbits) fill up from the inside to the out, and don't fill in the out ...
... Although Neils Bohr put the electrons into orbits, which was wrong, he did put them into energy levels. These levels are exact, and the electrons tend to stay in the lowest energy levels possible. That means that the orbitals (his orbits) fill up from the inside to the out, and don't fill in the out ...
Atomic Structure - Tumwater School District
... 2. All atoms of the same elements are identical. Atoms of different elements must be different. 3. Atoms of different elements can be combined together in ratios to form compounds. 4. Chemical reactions occur when atoms are separated, attached, or rearranged. But atoms aren’t changed, only rearrange ...
... 2. All atoms of the same elements are identical. Atoms of different elements must be different. 3. Atoms of different elements can be combined together in ratios to form compounds. 4. Chemical reactions occur when atoms are separated, attached, or rearranged. But atoms aren’t changed, only rearrange ...
Quantum Atom:
... this region related to the orbital Ψ2 gives probable electron density Transition absorb energy electron change orbitals and has higher energy Use results of hydrogen atom for other atoms Calculation predicts location in space in which electron is most likely found and energy of electron Orbitals l ...
... this region related to the orbital Ψ2 gives probable electron density Transition absorb energy electron change orbitals and has higher energy Use results of hydrogen atom for other atoms Calculation predicts location in space in which electron is most likely found and energy of electron Orbitals l ...
Unit 1: Introduction to Chemistry Directions: Use your notes
... Directions: Use your notes, power point lesson, and videos to help you answer the following questions. If you are unsure of an answer you can email me [email protected] or chat on Remind. Scientific Theories Dalton theorized that atoms were the smallest particle and could not be divided. ...
... Directions: Use your notes, power point lesson, and videos to help you answer the following questions. If you are unsure of an answer you can email me [email protected] or chat on Remind. Scientific Theories Dalton theorized that atoms were the smallest particle and could not be divided. ...
Build An Atom - cloudfront.net
... 1. You will be assigned an element. 2. You will then: * On a separate sheet of paper you will list everything you know about protons, neutrons, and electrons, and their behavior for your particular element. You will also draw the atomic structure for that element. * You will then create a model of y ...
... 1. You will be assigned an element. 2. You will then: * On a separate sheet of paper you will list everything you know about protons, neutrons, and electrons, and their behavior for your particular element. You will also draw the atomic structure for that element. * You will then create a model of y ...
Atoms, Elements, and Compounds
... In 1917, Rutherford made another discovery. He bombarded nitrogen gas with alpha particles and found that occasionally an oxygen atom was produced. He concluded that the alpha particles must have knocked a positively charged particle (which he named the proton) from the nucleus. He called this “play ...
... In 1917, Rutherford made another discovery. He bombarded nitrogen gas with alpha particles and found that occasionally an oxygen atom was produced. He concluded that the alpha particles must have knocked a positively charged particle (which he named the proton) from the nucleus. He called this “play ...
Summary 1 b - Uddingston Grammar School
... to remove further moles of electrons. 13. Explain the trends in first ionisation energy across periods and down groups in terms of atomic size, nuclear charge and the screening effect due to inner shell electrons. 14. Understand that atoms of different elements have different attractions for bonding ...
... to remove further moles of electrons. 13. Explain the trends in first ionisation energy across periods and down groups in terms of atomic size, nuclear charge and the screening effect due to inner shell electrons. 14. Understand that atoms of different elements have different attractions for bonding ...
Chemistry: Matter and Change
... radioactive decay. • Unstable radioactive elements undergo radioactive decay thus forming stable nonradioactive elements. ...
... radioactive decay. • Unstable radioactive elements undergo radioactive decay thus forming stable nonradioactive elements. ...
7R CHEMISTRY 1 REVIEW
... 2. If an element is divided into smaller and smaller parts, the smallest particle obtained would be a (an) A) molecule. B) compound. C) mixture. D) atom. 3. The fact that iron cannot be changed into a simpler form indicates that iron is a (an) A) compound. B) molecule. C) element. ...
... 2. If an element is divided into smaller and smaller parts, the smallest particle obtained would be a (an) A) molecule. B) compound. C) mixture. D) atom. 3. The fact that iron cannot be changed into a simpler form indicates that iron is a (an) A) compound. B) molecule. C) element. ...
Symbols of Elements - Chemistry with Mr. Patmos
... in order of increasing atomic mass He then arranged columns so that elements with most similar properties were next to each other – First Periodic table Blank spaces left bc/ no known elements with mass and properties that fit 1923 Henry Moseley determined atomic number of elements. Arranged e ...
... in order of increasing atomic mass He then arranged columns so that elements with most similar properties were next to each other – First Periodic table Blank spaces left bc/ no known elements with mass and properties that fit 1923 Henry Moseley determined atomic number of elements. Arranged e ...