Subatomic Particles - Ciencias Esmeralda
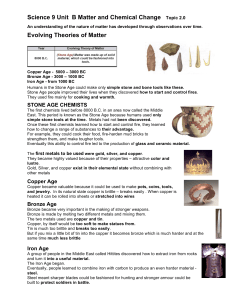
... If you round the atomic mass it gives you the mass number for the most common isotope. Unit is amu (atomic mass unit) 1 amu is 1/12 the mass of C-12 Gram atomic mass= amu but in grams ...
... If you round the atomic mass it gives you the mass number for the most common isotope. Unit is amu (atomic mass unit) 1 amu is 1/12 the mass of C-12 Gram atomic mass= amu but in grams ...
Chapter 4 Practice Test
... All atoms are ____. a. positively charged, with the number of protons exceeding the number of electrons b. negatively charged, with the number of electrons exceeding the number of protons c. neutral, with the number of protons equaling the number of electrons d. neutral, with the number of protons e ...
... All atoms are ____. a. positively charged, with the number of protons exceeding the number of electrons b. negatively charged, with the number of electrons exceeding the number of protons c. neutral, with the number of protons equaling the number of electrons d. neutral, with the number of protons e ...
BM 1 - answer key - Annapolis High School
... Mendeleev said that the properties of the elements are periodic if elements are arranged by increasing atomic mass. The use of mass was incorrect as Mendeleev found with the discovery of reversed pairs. Modern periodic law says the properties are periodic (and elements are in the same column if they ...
... Mendeleev said that the properties of the elements are periodic if elements are arranged by increasing atomic mass. The use of mass was incorrect as Mendeleev found with the discovery of reversed pairs. Modern periodic law says the properties are periodic (and elements are in the same column if they ...
Defining the Atom
... a. teaching that all matter is composed of tiny particles called atoms. b. theorizing that all atoms of the same element are identical. c. using experimental methods to establish a scientific theory. d. not relating atoms to chemical change. ...
... a. teaching that all matter is composed of tiny particles called atoms. b. theorizing that all atoms of the same element are identical. c. using experimental methods to establish a scientific theory. d. not relating atoms to chemical change. ...
Chapter 7 Honors Chemistry
... No two electrons in the same atom can have the same set of 4 quantum numbers. That is, each electron has a unique “address” In other words, the maximum # of electrons an orbital can hold is 2 e- (one with ms = +1/2 and one with ms = -1/2) ...
... No two electrons in the same atom can have the same set of 4 quantum numbers. That is, each electron has a unique “address” In other words, the maximum # of electrons an orbital can hold is 2 e- (one with ms = +1/2 and one with ms = -1/2) ...
Atomic Structure and the Periodic Table Continued
... neutrons mean for the element? This means the different isotopes of the same element have different masses!!!! Average Atomic Mass: ...
... neutrons mean for the element? This means the different isotopes of the same element have different masses!!!! Average Atomic Mass: ...
Atom - OCCC.edu
... extremely small, indivisible, indestructible particles called atoms. We now know that atoms are not ...
... extremely small, indivisible, indestructible particles called atoms. We now know that atoms are not ...
Chapter 2 Law of Conservation of Mass Law of Conservation of Mass
... 69. An unknown element (X) discovered on a planet in another galaxy was found to exist as two isotopes. Their atomic masses and percent abundances are listed in the following table. What is the relative atomic mass of the element? ...
... 69. An unknown element (X) discovered on a planet in another galaxy was found to exist as two isotopes. Their atomic masses and percent abundances are listed in the following table. What is the relative atomic mass of the element? ...
1 | Page Chemistry Lecture #19: Atomic Number, Isotopes, and
... elements could then be analyzed to reveal the number of protons in the nucleus. It was found that different elements had different numbers of protons. ...
... elements could then be analyzed to reveal the number of protons in the nucleus. It was found that different elements had different numbers of protons. ...
Unit2 PPT 3 Atomic Structure
... Protons and neutrons live compacted in the tiny positively charged nucleus accounting for more than 99% of the mass of the atom The negatively charged electrons are small and have a relatively small mass but occupy a large volume of space outside the nucleus ...
... Protons and neutrons live compacted in the tiny positively charged nucleus accounting for more than 99% of the mass of the atom The negatively charged electrons are small and have a relatively small mass but occupy a large volume of space outside the nucleus ...
chemistry notes: atomic structure
... • the smallest particle of an element retaining the properties of that element A. early theories and ideas, pro and con 1) Democritus of Abdera (460-370 B.C.): first atomic theory of matter • “atoma” / “atomos”—indivisible, indestructible particles in matter 2) Aristotle (384-322 B.C.): did not beli ...
... • the smallest particle of an element retaining the properties of that element A. early theories and ideas, pro and con 1) Democritus of Abdera (460-370 B.C.): first atomic theory of matter • “atoma” / “atomos”—indivisible, indestructible particles in matter 2) Aristotle (384-322 B.C.): did not beli ...
File
... – hypothesized that the chemistry of the elements might be related to their masses; – arranged the known elements in order of increasing atomic masses; – discovered that every 7th element had similar properties (noble gases were still unknown during this time); – Proposed the arrangement of elements ...
... – hypothesized that the chemistry of the elements might be related to their masses; – arranged the known elements in order of increasing atomic masses; – discovered that every 7th element had similar properties (noble gases were still unknown during this time); – Proposed the arrangement of elements ...
Honors Chemistry
... Which of the following is not part of Dalton’s Atomic Theory? a. Atoms of a given element have the same mass. b. All elements are made of indivisible, indestructible atoms. c. Atoms of one element can be converted into a different element. d. Compounds are the result of the combination of atoms of d ...
... Which of the following is not part of Dalton’s Atomic Theory? a. Atoms of a given element have the same mass. b. All elements are made of indivisible, indestructible atoms. c. Atoms of one element can be converted into a different element. d. Compounds are the result of the combination of atoms of d ...
Ch. 4 Statement of Evidence Atoms + Notes Outline
... 4. How do negative charges of particles within an atom react with positive charges? (pg. 84) Opposites attract-so negative particles attract towards positive particles. 5. How do like charges of particles in an atom react? (pg. 86) Like charged particles repel/push away (negative repel negative) (po ...
... 4. How do negative charges of particles within an atom react with positive charges? (pg. 84) Opposites attract-so negative particles attract towards positive particles. 5. How do like charges of particles in an atom react? (pg. 86) Like charged particles repel/push away (negative repel negative) (po ...
What is the Matter?
... • The top number is the atomic number. The atomic number tells how many protons are in one atom of that element. • The bigger number is the atomic mass. The atomic mass is the sum of the protons, neutrons, and electrons. ...
... • The top number is the atomic number. The atomic number tells how many protons are in one atom of that element. • The bigger number is the atomic mass. The atomic mass is the sum of the protons, neutrons, and electrons. ...
File
... have come from inside atoms. So Dalton's idea of the indestructible atom had to be revised. Thomson proposed a different model for the atom. He said that the tiny negatively charged electrons must be embedded in a cloud of positive charge (after all, atoms themselves carry no overall charge, so the ...
... have come from inside atoms. So Dalton's idea of the indestructible atom had to be revised. Thomson proposed a different model for the atom. He said that the tiny negatively charged electrons must be embedded in a cloud of positive charge (after all, atoms themselves carry no overall charge, so the ...
Unit 1: Introduction to Chemistry
... 52. First, the _sublevel_ is written, followed by _superscript_ indicating… ...
... 52. First, the _sublevel_ is written, followed by _superscript_ indicating… ...
No Slide Title
... Reading and Writing Think of a memory chip as a grid or array of capacitors located at specific rows and columns. If we choose to read the memory cell located at row 3, column 5, we will retrieve information from a specific capacitor. Every time we go to row 3, column 5, we will access or address ...
... Reading and Writing Think of a memory chip as a grid or array of capacitors located at specific rows and columns. If we choose to read the memory cell located at row 3, column 5, we will retrieve information from a specific capacitor. Every time we go to row 3, column 5, we will access or address ...
chemistry 1
... Elements and Isotopes A chemical element is a pure substance that consists entirely of one type of atom. More than 100 elements are known, but only about two dozen are commonly found in living organisms. Elements are represented by one- or two-letter symbols. For example, C stands for carbon, H for ...
... Elements and Isotopes A chemical element is a pure substance that consists entirely of one type of atom. More than 100 elements are known, but only about two dozen are commonly found in living organisms. Elements are represented by one- or two-letter symbols. For example, C stands for carbon, H for ...