Steve Hansen`s second test - Kwantlen Polytechnic University
... The energy required to dissociate H2 molecules into H atoms is 432 kJ/mol. If the dissociation of an H2 molecule was accompolished by the absorption of a single photon with exactly the energy required, what would be its wavelength (in nanometers)? (4) ...
... The energy required to dissociate H2 molecules into H atoms is 432 kJ/mol. If the dissociation of an H2 molecule was accompolished by the absorption of a single photon with exactly the energy required, what would be its wavelength (in nanometers)? (4) ...
Bonding Notes
... MgCl2 tells you that these salt crystals have 1 magnesium ion for each two chloride ions In general, for any pair of positive and negative ions there is only one ratio and, thus, one formula that results in overall electrical neutrality. ...
... MgCl2 tells you that these salt crystals have 1 magnesium ion for each two chloride ions In general, for any pair of positive and negative ions there is only one ratio and, thus, one formula that results in overall electrical neutrality. ...
Nickel 28 Ni 58.693
... a polar molecule and the molecule will have a bent shape. positive and negative ions ...
... a polar molecule and the molecule will have a bent shape. positive and negative ions ...
ionic bond. - cloudfront.net
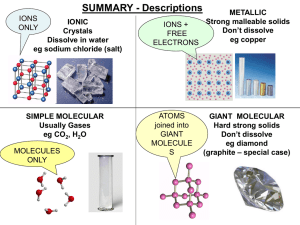
... • Metals- conduct heat, have low ionization energy • Low EN; give up electrons easily. • Metals have luster (shine), are malleable (can be hammered into sheets) and are ductile (drawn into wires). ...
... • Metals- conduct heat, have low ionization energy • Low EN; give up electrons easily. • Metals have luster (shine), are malleable (can be hammered into sheets) and are ductile (drawn into wires). ...
Bonding Nomenclature Notes
... 3) Add prefixes to both indicating the number of atoms of each element ...
... 3) Add prefixes to both indicating the number of atoms of each element ...
Apply the octet rule to atoms that form covalent bonds
... Apply the octet rule to atoms that form covalent bonds. Describe the formation of single, double, and triple covalent bonds. ...
... Apply the octet rule to atoms that form covalent bonds. Describe the formation of single, double, and triple covalent bonds. ...
Atomic Structure, Molecular Structure & Bonding
... 4. Count ve-’s and compare to #2 5. If too many e-’s, make a double bond 6. Calculate formal charge (FC) to double check structure – No or low FCs (e.g. +1) more likely than large FCs (e.g. ...
... 4. Count ve-’s and compare to #2 5. If too many e-’s, make a double bond 6. Calculate formal charge (FC) to double check structure – No or low FCs (e.g. +1) more likely than large FCs (e.g. ...
chapter02_part1_lecture - bloodhounds Incorporated
... Molecules usually form when two or more atoms bond together by forming covalent bonds (example: O2) A molecule is the smallest particle of matter that can have independent existence: He, Ne, H2, N2, O2, Cl2, CO2, H2O, NH3, CH4 ...
... Molecules usually form when two or more atoms bond together by forming covalent bonds (example: O2) A molecule is the smallest particle of matter that can have independent existence: He, Ne, H2, N2, O2, Cl2, CO2, H2O, NH3, CH4 ...
Chapter 2 part 1
... Molecules usually form when two or more atoms bond together by forming covalent bonds (example: O2) A molecule is the smallest particle of matter that can have independent existence: He, Ne, H2, N2, O2, Cl2, CO2, H2O, NH3, CH4 ...
... Molecules usually form when two or more atoms bond together by forming covalent bonds (example: O2) A molecule is the smallest particle of matter that can have independent existence: He, Ne, H2, N2, O2, Cl2, CO2, H2O, NH3, CH4 ...
Test Specs - Blue Valley Schools
... configurations, ionization energy, and atomic radius. 5. Predict the electron configuration for an element based on the number of electrons. 6. Predict the electron configuration for an element based on the location on the periodic table. 7. Identify a few exceptions to the electron configuration ru ...
... configurations, ionization energy, and atomic radius. 5. Predict the electron configuration for an element based on the number of electrons. 6. Predict the electron configuration for an element based on the location on the periodic table. 7. Identify a few exceptions to the electron configuration ru ...
Lecture 2 - The Dionne Group
... shared electrons. The four bonds are identical and repel each other. In three dimensions, due to symmetry, the bonds are directed towards the corners of a tetrahedron. ...
... shared electrons. The four bonds are identical and repel each other. In three dimensions, due to symmetry, the bonds are directed towards the corners of a tetrahedron. ...
NOTES: 2.1 - Intro to Chemistry
... ● a MOLECULE is the smallest unit of most compounds! ● EXAMPLE: 1 molecule of water, H2O, is the smallest unit of water possible; it consists of 2 hydrogen atoms & 1 oxygen atom bonded together. ...
... ● a MOLECULE is the smallest unit of most compounds! ● EXAMPLE: 1 molecule of water, H2O, is the smallest unit of water possible; it consists of 2 hydrogen atoms & 1 oxygen atom bonded together. ...
Atoms and Elements Notes
... 2. Do not react with anything due to having 8 valence electrons 3. All gases ...
... 2. Do not react with anything due to having 8 valence electrons 3. All gases ...
File
... • atoms that are covalently bonded form molecules • when two atoms form a covalent bond the sharing of electrons allows each to satisfy the octet rule ...
... • atoms that are covalently bonded form molecules • when two atoms form a covalent bond the sharing of electrons allows each to satisfy the octet rule ...
Chapter 5
... valence electrons for main group elements alkali metals, alkali earth metals, halogens, noble gases metals, nonmetals, metalloids (semimetals); general properties and location Effective nuclear charge, Zeff; approximate value for Zeff, calculation and interpretation Zeff and Coulomb’s law Trends in ...
... valence electrons for main group elements alkali metals, alkali earth metals, halogens, noble gases metals, nonmetals, metalloids (semimetals); general properties and location Effective nuclear charge, Zeff; approximate value for Zeff, calculation and interpretation Zeff and Coulomb’s law Trends in ...
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electrostatic force of attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction. The strength of chemical bonds varies considerably; there are ""strong bonds"" such as covalent or ionic bonds and ""weak bonds"" such as Dipole-dipole interaction, the London dispersion force and hydrogen bonding.Since opposite charges attract via a simple electromagnetic force, the negatively charged electrons that are orbiting the nucleus and the positively charged protons in the nucleus attract each other. An electron positioned between two nuclei will be attracted to both of them, and the nuclei will be attracted toward electrons in this position. This attraction constitutes the chemical bond. Due to the matter wave nature of electrons and their smaller mass, they must occupy a much larger amount of volume compared with the nuclei, and this volume occupied by the electrons keeps the atomic nuclei relatively far apart, as compared with the size of the nuclei themselves. This phenomenon limits the distance between nuclei and atoms in a bond.In general, strong chemical bonding is associated with the sharing or transfer of electrons between the participating atoms. The atoms in molecules, crystals, metals and diatomic gases—indeed most of the physical environment around us—are held together by chemical bonds, which dictate the structure and the bulk properties of matter.All bonds can be explained by quantum theory, but, in practice, simplification rules allow chemists to predict the strength, directionality, and polarity of bonds. The octet rule and VSEPR theory are two examples. More sophisticated theories are valence bond theory which includes orbital hybridization and resonance, and the linear combination of atomic orbitals molecular orbital method which includes ligand field theory. Electrostatics are used to describe bond polarities and the effects they have on chemical substances.