Unit - eBoard
... Covalent – Polar & Nonpolar Drawing and labeling Polar & Nonpolar compounds Metallic Video: World of Chemistry – Chemical Bonding Periodic Table of Electronegativity Using Electronegativity Difference to determine bond type Ionic Bonding & Ionic Compounds Formation of Ionic Bonds Formula Unit Crysta ...
... Covalent – Polar & Nonpolar Drawing and labeling Polar & Nonpolar compounds Metallic Video: World of Chemistry – Chemical Bonding Periodic Table of Electronegativity Using Electronegativity Difference to determine bond type Ionic Bonding & Ionic Compounds Formation of Ionic Bonds Formula Unit Crysta ...
PYP001-121 Major-I Solution. In all the questions, choice
... Q1. Which of the following statements is FALSE? A) Smoke is a compound. B) A pure substance can be either an element or compound. C) A fruit salad is a heterogeneous mixture. D) Every type of atom has a different number of protons. E) The change of state from a gas to a liquid is called condensation ...
... Q1. Which of the following statements is FALSE? A) Smoke is a compound. B) A pure substance can be either an element or compound. C) A fruit salad is a heterogeneous mixture. D) Every type of atom has a different number of protons. E) The change of state from a gas to a liquid is called condensation ...
Midterm Review Answers
... Questions 52-53nrefer to the following types of energy A) Activation energy B) Free energy C) Ionization energy D) Kinetic energy E) Lattice energy 52. The energy required to convert a ground-state atom in the gas phase to a gaseous positive ion. C 53. The energy released when gas phase ions bond t ...
... Questions 52-53nrefer to the following types of energy A) Activation energy B) Free energy C) Ionization energy D) Kinetic energy E) Lattice energy 52. The energy required to convert a ground-state atom in the gas phase to a gaseous positive ion. C 53. The energy released when gas phase ions bond t ...
Sections 6.4 - 6.5
... This introduces E=O bonds, which due to their electron withdrawing effect increase the polarization of the E --O-H + bond system further → acidic reaction in water. ...
... This introduces E=O bonds, which due to their electron withdrawing effect increase the polarization of the E --O-H + bond system further → acidic reaction in water. ...
Ch 2 Atomic History
... Results: Most of the α particles went straight through the foil without deflection. Some α-particles were deflected at high angles. ...
... Results: Most of the α particles went straight through the foil without deflection. Some α-particles were deflected at high angles. ...
CH 6 electrons in atoms
... 2) In each orbit of radius r, the angular momentum of the electron (mevr, [mass, velocity, radius]) is restricted to values of (nh/2π), where n is a whole number. This means the electron is quantized. 3) When an electron moves from one orbit to another, the energy difference, ∆E, between the two orb ...
... 2) In each orbit of radius r, the angular momentum of the electron (mevr, [mass, velocity, radius]) is restricted to values of (nh/2π), where n is a whole number. This means the electron is quantized. 3) When an electron moves from one orbit to another, the energy difference, ∆E, between the two orb ...
Chemical Reactions
... Notice that there are two hydrogen atoms on each side however there are two oxygen atoms in the reactants but only one in the products. To balance this we must insert a coefficient. H2 + ...
... Notice that there are two hydrogen atoms on each side however there are two oxygen atoms in the reactants but only one in the products. To balance this we must insert a coefficient. H2 + ...
Van der Waals Forces Between Atoms
... The perfect gas equation of state PV = NkT is manifestly incapable of describing actual gases at low temperatures, since they undergo a discontinuous change of volume and become liquids. In the 1870’s, the Dutch physicist Van der Waals came up with an improvement: a gas law that recognized the m ...
... The perfect gas equation of state PV = NkT is manifestly incapable of describing actual gases at low temperatures, since they undergo a discontinuous change of volume and become liquids. In the 1870’s, the Dutch physicist Van der Waals came up with an improvement: a gas law that recognized the m ...
Budiansky Cover
... Panza’s little had forgotten and the good beast molecules?” donkey came from. reappeared. ...
... Panza’s little had forgotten and the good beast molecules?” donkey came from. reappeared. ...
How many significant figures are there in each of these
... - Dalton's theory sets LIMITS on what can be done with chemistry. For example: Chemistry can't convert lead (an element) into gold (another element). Sorry, alchemists! You can't have a compound form in a chemical reaction that contains an element that was not in your starting materials. You can onl ...
... - Dalton's theory sets LIMITS on what can be done with chemistry. For example: Chemistry can't convert lead (an element) into gold (another element). Sorry, alchemists! You can't have a compound form in a chemical reaction that contains an element that was not in your starting materials. You can onl ...
Unit 3: Bonding and Nomenclature Content Outline: Chemical
... For example: H20 (water) or O2 (Oxygen gas) or C6H12O6 (Glucose) B. This term is usually used with molecules that are bound together using covalent bonds. C. These molecules can possess single bonds (-), double bonds (=), or even triple bonds (Ξ). 1. The purpose of “creating” the bonds is to achieve ...
... For example: H20 (water) or O2 (Oxygen gas) or C6H12O6 (Glucose) B. This term is usually used with molecules that are bound together using covalent bonds. C. These molecules can possess single bonds (-), double bonds (=), or even triple bonds (Ξ). 1. The purpose of “creating” the bonds is to achieve ...
Notes for Matter Packet- Balancing equations (PDF
... – Chemical reactions occur when bonds between the outermost parts of atoms are formed or broken – Chemical reactions involve changes in matter, the making of new materials with new properties, and energy changes. – Symbols represent elements, formulas describe compounds, chemical equations describe ...
... – Chemical reactions occur when bonds between the outermost parts of atoms are formed or broken – Chemical reactions involve changes in matter, the making of new materials with new properties, and energy changes. – Symbols represent elements, formulas describe compounds, chemical equations describe ...
atoms
... Consider the compound water made up of two atoms of hydrogen (H) for every atoms of oxygen (O) Can be presented chemical formula H20 Two samples describes below have the same proportions of the two ...
... Consider the compound water made up of two atoms of hydrogen (H) for every atoms of oxygen (O) Can be presented chemical formula H20 Two samples describes below have the same proportions of the two ...
atoms
... Alpha (a): a-particles carry two fundamental units of positive charge and the same mass as helium atoms. This particle are identical to He2+ions Beta (b): b-particles are negatively charged and have the same properties as electrons Gamma (g) rays: is not effected by electric or magnetic field. ...
... Alpha (a): a-particles carry two fundamental units of positive charge and the same mass as helium atoms. This particle are identical to He2+ions Beta (b): b-particles are negatively charged and have the same properties as electrons Gamma (g) rays: is not effected by electric or magnetic field. ...
Unit 3 Test - hrsbstaff.ednet.ns.ca
... ___ Combustibility is the ability of a substance to react with acids ___ Sugar disappearing in water is an example of a solution ___ Raisins in Raisin Bran are an example of a solution ___ Lighting a test tube of acetylene gas is an example of a reaction with acid ___ Lighting a test tube of acetyle ...
... ___ Combustibility is the ability of a substance to react with acids ___ Sugar disappearing in water is an example of a solution ___ Raisins in Raisin Bran are an example of a solution ___ Lighting a test tube of acetylene gas is an example of a reaction with acid ___ Lighting a test tube of acetyle ...
Document
... Atoms gain electrons (negatives) and become more negative. Atoms with 2-3 valence electrons will LOSE electrons and become more positive. Who will lose and who will gain an electron? ...
... Atoms gain electrons (negatives) and become more negative. Atoms with 2-3 valence electrons will LOSE electrons and become more positive. Who will lose and who will gain an electron? ...
PPT - kimscience.com
... All atoms of a given element are identical in their physical and chemical properties; they differ from atoms of every other element Atoms of different elements combine in simple whole-number ratios to form compounds (can form more than one compound together) Chemical reactions consist of the comb ...
... All atoms of a given element are identical in their physical and chemical properties; they differ from atoms of every other element Atoms of different elements combine in simple whole-number ratios to form compounds (can form more than one compound together) Chemical reactions consist of the comb ...
Chapter One Outline
... composition of a substance. Examples include temperature, mass, density, etc. Density is the ratio of an objects mass to its volume; D = m/v Chemical Properties A substances chemical properties describe the kinds of chemical reactions the substance can undergo Chemical reactions are usually accompan ...
... composition of a substance. Examples include temperature, mass, density, etc. Density is the ratio of an objects mass to its volume; D = m/v Chemical Properties A substances chemical properties describe the kinds of chemical reactions the substance can undergo Chemical reactions are usually accompan ...
Matter Vocab Part 4
... Particle in the nucleus of an atom with a positive charge Particle in the nucleus of an atom with no charge Particle orbiting the nucleus of an atom with a negative charge Dense, central core of an atom (made of protons and neutrons) Path an electron takes around the nucleus A shell is sometimes cal ...
... Particle in the nucleus of an atom with a positive charge Particle in the nucleus of an atom with no charge Particle orbiting the nucleus of an atom with a negative charge Dense, central core of an atom (made of protons and neutrons) Path an electron takes around the nucleus A shell is sometimes cal ...
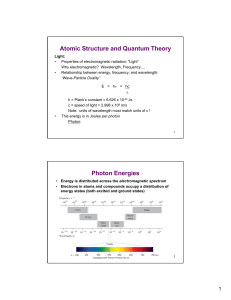
Atomic Structure and Quantum Theory Photon Energies
... – Electrons are small, constantly moving – Electrons occupy specific (quantized) levels in an atom – Electrons have properties of BOTH particles and waves – At any instant, it is impossible to pinpoint the position of an electron of a given energy with high accuracy. Because of this combination, the ...
... – Electrons are small, constantly moving – Electrons occupy specific (quantized) levels in an atom – Electrons have properties of BOTH particles and waves – At any instant, it is impossible to pinpoint the position of an electron of a given energy with high accuracy. Because of this combination, the ...
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electrostatic force of attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction. The strength of chemical bonds varies considerably; there are ""strong bonds"" such as covalent or ionic bonds and ""weak bonds"" such as Dipole-dipole interaction, the London dispersion force and hydrogen bonding.Since opposite charges attract via a simple electromagnetic force, the negatively charged electrons that are orbiting the nucleus and the positively charged protons in the nucleus attract each other. An electron positioned between two nuclei will be attracted to both of them, and the nuclei will be attracted toward electrons in this position. This attraction constitutes the chemical bond. Due to the matter wave nature of electrons and their smaller mass, they must occupy a much larger amount of volume compared with the nuclei, and this volume occupied by the electrons keeps the atomic nuclei relatively far apart, as compared with the size of the nuclei themselves. This phenomenon limits the distance between nuclei and atoms in a bond.In general, strong chemical bonding is associated with the sharing or transfer of electrons between the participating atoms. The atoms in molecules, crystals, metals and diatomic gases—indeed most of the physical environment around us—are held together by chemical bonds, which dictate the structure and the bulk properties of matter.All bonds can be explained by quantum theory, but, in practice, simplification rules allow chemists to predict the strength, directionality, and polarity of bonds. The octet rule and VSEPR theory are two examples. More sophisticated theories are valence bond theory which includes orbital hybridization and resonance, and the linear combination of atomic orbitals molecular orbital method which includes ligand field theory. Electrostatics are used to describe bond polarities and the effects they have on chemical substances.